 |
 |

Current Status of Testosterone Replacement Therapy in Men
Stephen J. Winters, MD
Arch Fam Med. 1999;8:257-263.
ABSTRACT
 |  |
Testosterone plays an essential role in the development of the normal male and in the maintenance of many male characteristics, including muscle mass and strength, bone mass, libido, potency, and spermatogenesis. Androgen deficiency occurs with disorders that damage the testes, including traumatic or surgical castration (primary testicular failure) or disorders in which the gonadotropin stimulation of the testes is reduced (hypogonadotropic hypogonadism). The clinical manifestations of androgen deficiency depend on the age at onset and the severity and duration of the deficiency. In adult males, these manifestations may include reduced body hair, decreased muscle mass and strength, increased fat mass, decreased hematocrit, decreased libido, erectile dysfunction, infertility, osteoporosis, and depressed mood. The forms of androgen replacement currently available in the United States are intramuscular depot injections of testosterone esters, oral tablets of testosterone derivatives, and transdermal patches. For most patients, androgen replacement therapy with testosterone is a safe, effective treatment for testosterone deficiency.
INTRODUCTION
Testosterone, the most important androgen produced by the testes, plays a crucial role in the health of the male. During fetal development, testosterone and its metabolite dihydrotestosterone (DHT) are needed for normal differentiation of male internal and external genitalia. During puberty, testosterone is required for the development of male secondary sexual characteristics, stimulation of sexual behavior and function, and initiation of sperm production.1 In adult males, testosterone maintains muscle mass and strength, fat distribution, bone mass, erythropoiesis, male hair pattern, libido and potency, and spermatogenesis.2
Circulating testosterone levels have a diurnal variation in normal young men, usually reaching a mean maximum level of 25 nmol/L (710 ng/dL) at approximately 8 AM and declining to a mean minimum level of 15 nmol/L (426 ng/dL) at approximately 10 PM.3-4 This circadian variation in testosterone level appears to be a result of temporal modulation of hormone secretion by the testes rather than of a diurnal change in testosterone clearance, although the precise mechanism is unknown. Circulating testosterone is metabolized to DHT in the skin, liver, prostate, and other organs that contain the enzyme 5 -reductase.2 Testosterone is also metabolized to estradiol (E2) by the aromatase enzyme complex in the brain, fat, and testes.2 In normal men the ratio of the resulting plasma levels of DHT and E2 to the total testosterone level are approximately 1:10 and 1:200, respectively. Typical circadian patterns of testosterone, DHT, and E2 in healthy young men are shown in Figure 1.4 These 3 steroids bind to and activate intracellular receptors that are specific for either androgens (testosterone and DHT) or estrogens. These receptors are found in the reproductive, immune, hematopoietic, and central nervous systems. Organs and tissues influenced include the pituitary, liver, kidneys, muscles, bones, adipose, and skin. -reductase.2 Testosterone is also metabolized to estradiol (E2) by the aromatase enzyme complex in the brain, fat, and testes.2 In normal men the ratio of the resulting plasma levels of DHT and E2 to the total testosterone level are approximately 1:10 and 1:200, respectively. Typical circadian patterns of testosterone, DHT, and E2 in healthy young men are shown in Figure 1.4 These 3 steroids bind to and activate intracellular receptors that are specific for either androgens (testosterone and DHT) or estrogens. These receptors are found in the reproductive, immune, hematopoietic, and central nervous systems. Organs and tissues influenced include the pituitary, liver, kidneys, muscles, bones, adipose, and skin.
|
|

|
|
Figure 1. Mean curves and range of circadian patterns of testosterone, dihydrotestosterone, and estradiol in healthy young men. Dashed lines represent the 95% confidence range for the estimated circadian patterns. Symbols correspond to mean curves reported in different studies. Adapted with permission from Mazer et al.4
|
|
|
CLINICAL MANIFESTATIONS OF ANDROGEN DEFICIENCY
Androgen deficiency, also known as hypogonadism, results from the subnormal production of testosterone by the testes. The prevalence of androgen deficiency is not known with certainty, and hypogonadism is probably underdiagnosed. Some common causes of hypogonadism are listed in Table 1. Testicular failure may have a genetic or a developmental basis, or may be acquired. Klinefelter syndrome (47,XXY), the most common cause of primary testicular failure, occurs in approximately 1 of 1000 newborn males.5-7 Hypogonadotropic (secondary) hypogonadism may result from either acquired or congenital defects in pituitary or hypothalamic function.8 The clinical manifestations of androgen deficiency depend on the age at onset and the severity and duration of the deficiency. Hypogonadism is diagnosed easily when the usual signs and symptoms of androgen deficiency are present (Table 2), or when the patient has a history of a predisposing condition such as mumps orchitis, orchiectomy, or irradiation to the pelvis or head. Conversely, the diagnosis can be more difficult in patients with less specific symptoms or an unremarkable clinical history. Fortunately, simple laboratory tests provide accurate information about levels of total and bioavailable testosterone and of gonadotropins. These tests should be performed in any male patient with symptoms suggestive of androgen deficiency.
|
|

|
|
Table 1. Disorders Causing Male Hypogonadism*
|
|
|
|
|

|
|
Table 2. Clinical Manifestations of Male Hypogonadism
|
|
|
Although the range of normal values varies among laboratories, morning testosterone values below 12 nmol/L (350 ng/dL) suggest hypogonadism and should be confirmed by a second determination. Testosterone levels fall gradually as men grow older, but most elderly men have testosterone levels that are in the low-normal range for younger men.9 In men older than 65 years, morning values below 9 nmol/L (250 ng/dL) should be investigated further. Approximately 50% of the circulating testosterone is tightly bound to sex hormone binding globulin produced by the liver, so that increased or decreased levels of sex hormone binding globulin influence the measured testosterone level. When borderline total testosterone values are found, or the clinical picture and the serum testosterone levels disagree, additional measures of circulating androgens are needed. The most accurate indicator of hypogonadism is the concentration of testosterone that is not bound to sex hormone binding globulin (the concentration of bioavailable testosterone or free testosterone).10 Men with hypogonadotropic hypogonadism have low plasma testosterone levels and luteinizing hormone levels that may be low or low-normal. Thus, the plasma level of follicle-stimulating hormone and luteinizing hormone should be measured. The serum prolactin levels should then be measured because hyperprolactinemia suggests the presence of a pituitary tumor.10 Additional hormone evaluations and imaging tests may be indicated. Primary testicular failure is accompanied by elevated plasma levels of follicle-stimulating hormone and luteinizing hormone because of impaired negative feedback inhibition of gonadotropin secretion.
TESTOSTERONE REPLACEMENT THERAPY
With few exceptions, confirmed hypogonadism requires testosterone replacement. Benefits of androgen replacement therapy include increased body hair and beard growth, energy, hematocrit, muscle mass, strength and stamina; increased ability to perform more physically demanding tasks; and an overall increase in the sense of well-being, confidence, and motivation.1 Untreated hypogonadism is a prominent cause of osteoporosis in men.11 Bone mineral density is increased by testosterone replacement in hypogonadal men,12 and the concomitant increase in muscle mass and strength may help prevent falls that predispose older men to fractures. Hypogonadal men treated with androgens experience improved libido and sexual function, as indicated by frequency of erection and ejaculation.13-16 In sexually immature eunuchoidal men, androgen replacement therapy promotes the development of secondary sexual characteristics. If the epiphyses are still open, androgen therapy promotes longitudinal bone growth until epiphyseal closure.
Although the benefits of androgen replacement therapy are clear, the delivery of testosterone to hypogonadal men in a way that approximates normal patterns and levels poses a therapeutic challenge. Much effort has been devoted to developing the ideal androgen replacement therapy. It is generally agreed that such therapy would deliver physiological amounts (3-10 mg/d) of testosterone; produce consistent levels of testosterone, DHT, and E2 within normal physiological ranges; and mimic the circadian patterns of hormone levels found in healthy young men. It would have a good safety profile without adverse effects on the prostate, serum lipids, liver, or respiratory function. Finally, it would be "patient-friendly." Parenteral, oral, and transdermal formulations of testosterone are currently available (Table 3).
|
|

|
|
Table 3. Summary of Available Androgen Replacement Therapies*
|
|
|
Natural Testosterone
Natural testosterone is not available commercially in the United States, but if prescribed by a physician, it can be compounded and sold by pharmacists. Natural testosterone administered orally or sublingually is rapidly and extensively metabolized by the liver. For example, buccal administration of natural testosterone at a dose of 10 mg produced peak levels of 94 nmol/L (2700 ng/dL) 30 minutes after dosing, with a return to baseline levels by 4 to 6 hours.17 Twice-daily treatment for 8 weeks increased sexual function, assessed by nocturnal penile tumescence testing in a sleep laboratory, compared with placebo in middle-aged men with hypogonadism, but there was substantial overlap between the groups in subjective end points such as libido and overall sexual function. The wide fluctuations in the plasma testosterone level may produce emotional lability, and, most importantly, the long-term consequences of the very high peak values that occur immediately after dosing are unknown. Moreover, it is difficult to monitor therapy by measuring the testosterone level in plasma, or to determine how many daily doses represent physiological replacement. Therefore, a more constant mode of hormone delivery seems advantageous.
Intramuscular Depot Injections
The most commonly used forms of androgen replacement therapy have been intramuscular depot injections of the testosterone esters, testosterone enanthate and testosterone cypionate, in an oil suspension. Esterification increases the lipid solubility of testosterone and prolongs its action. The esters are converted to free testosterone in the circulation. Although it is important that dosages be adjusted to meet the needs of the individual patient, the usual dosage for adults is 150 to 200 mg administered every 14 to 21 days. This regimen is usually successful in maintaining normal androgenization without marked adverse effects.
A major disadvantage of intramuscular administration of testosterone esters is the high levels of serum testosterone produced for several days after injection, and low or subnormal levels resulting at the end of the dosing interval (Figure 2).8, 18 These profiles may be accompanied by disturbing fluctuations in sexual function, energy level, and mood.1 Supraphysiologic levels of testosterone may predispose the patient to acne and polycythemia and result in high postinjection estradiol levels and gynecomastia.19 In some patients, injections may be associated with local pain, bleeding, or bruising, and with allergic reactions to sesame oil, the injection vehicle for testosterone enanthate, or to cottonseed oil, the vehicle for testosterone cypionate.19 Although self-administration is possible, many patients visit the physician's office or clinic for treatment, a process that may be expensive and inconvenient.
|
|

|
|
Figure 2. Steady-state pharmacokinetic profiles of testosterone in hypogonadal men treated with intramuscular testosterone enanthate at a dosage of 200 mg every 2 weeks. The horizontal lines represent the lower and upper boundaries of the normal range. Adapted with permission from Snyder and Lawrence.18
|
|
|
Alkylated Androgens
Several alkylated derivatives of testosterone are available for oral or sublingual use, including methyltestosterone and fluoxymesterone. Alkylated androgens are more slowly metabolized by the liver than is natural testosterone, but, like testosterone, these androgens interact directly with androgen receptors. Although their oral route of administration is advantageous, clinical response is variable20 and plasma levels cannot be determined, because alkylated androgens are not recognized by most testosterone assays. Moreover, in our clinical experience, alkylated androgens may increase levels of low-density lipoprotein cholesterol and profoundly suppress high-density lipoprotein cholesterol levels because of their route of absorption and metabolism.21 Prolonged use of high doses of androgens (principally the 17 -alkylated androgens) has been associated with development of the following potentially life-threatening conditions: hepatic adenomas, hepatocellular carcinoma, and peliosis hepatis. Cholestatic hepatitis and jaundice may occur at relatively low doses of 17 -alkylated androgens) has been associated with development of the following potentially life-threatening conditions: hepatic adenomas, hepatocellular carcinoma, and peliosis hepatis. Cholestatic hepatitis and jaundice may occur at relatively low doses of 17 -alkylated androgens.22(pp1263,2614) -alkylated androgens.22(pp1263,2614)
Transdermal Testosterone Replacement
Although it acts as a barrier to noxious agents, the skin absorbs some drugs, including steroid hormones, into the systemic circulation. Transdermal administration delivers testosterone at a controlled rate into the systemic circulation, avoiding the high and low levels observed with long-acting testosterone injections.Because scrotal skin is at least 5 times more permeable to testosterone than are other skin sites, the first available testosterone transdermal delivery system (Testoderm; Alza Pharmaceuticals, Palo Alto, Calif) was designed as a scrotal patch. Patients using the scrotal testosterone system have reported substantially improved sexual function, including the achievement of potency, and an improvement in sense of well-being, mood, and energy.23-24 Although testosterone is delivered at a controlled rate, scrotal patches have been associated with high levels of DHT because of the presence of the enzyme 5 -reductase in scrotal skin, which results in a high rate of testosterone metabolism.24 The patch may be irritating or difficult to keep in place, however, and requires dry shaving of the scrotum before application. Use of scrotal patches is not feasible if the scrotal surface is inadequate. -reductase in scrotal skin, which results in a high rate of testosterone metabolism.24 The patch may be irritating or difficult to keep in place, however, and requires dry shaving of the scrotum before application. Use of scrotal patches is not feasible if the scrotal surface is inadequate.
Two transdermal systems for the delivery of testosterone across nonscrotal skin have been developed. Androderm (SmithKline Beecham Pharmaceuticals, Philadelphia, Pa) is a liquid reservoir system with a permeation-enhancing vehicle of ethanol, water, monoglycerides, fatty acid esters, and gelling agents25 that allows absorption of testosterone through nonscrotal skin. Patches that deliver natural testosterone in amounts of 2.5 or 5 mg/d are available with a surface area of 44 cm2 or 74 cm2, respectively. For most men, one 5-mg patch is applied each night, rotating among various sites on the back, abdomen, upper arms, and thighs. A few big men require a dose of 7.5 mg to achieve normal circulating testosterone levels. The 2.5-mg system is useful for teenagers. Serum testosterone levels with a normal diurnal variation and normal plasma levels of DHT and E2 are produced (Figure 3).26 Improvements in sexual function, libido, energy level, and mood have been reported by patients after using the nonscrotal transdermal system.25, 27 The incidence of polycythemia is lower than in men treated with testosterone enanthate, 200 mg every 2 weeks. The nonscrotal patch eliminates the technical problems that may occur with the scrotal system, ie, inadequate scrotal surface and dry shaving of the scrotum.
|
|

|
|
Figure 3. Plasma testosterone concentrations during 24 hours after the application of two 37-cm2 permeation-enhanced transdermal patches applied nightly at 10 PM. Adapted from Meikle et al.26 Used by permission of Wiley-Liss, Inc, a subsidiary of John Wiley & Sons, Inc.
|
|
|
Local skin reactions are the most common adverse events reported for the nonscrotal testosterone transdermal system, with approximately 50% of men who participated in clinical trials reporting transient, mild to moderate erythema occurring at the application site sometime during therapy.26 Generalized allergic dermatitis that required discontinuation of therapy occurred occasionally. Burnlike blister reactions occurred in 12% of men during the clinical trials, typically only once at a single application site. These reactions occurred at a rate of 1 in 6500 system applications and did not lead to discontinuation of treatment. Most of these reactions were associated with application of the systems over bony prominences or on parts of the body that could have been subject to prolonged pressure during sleep or sitting. Recommended sites for system application include the back, abdomen, upper arms, and thighs. Pretreatment of the application site with 0.1% triamcinolone acetonide cream decreases the skin reactions resulting from this system.28
In clinical trials of up to 12 months' duration, mean serum prostate-specific antigen levels and mean prostate volume as estimated by transrectal ultrasound remained within the normal range. Safety assessments have disclosed no clinically significant changes in lipid measures or results of serum chemistry studies.26-27
Testoderm TTS for application to nonscrotal skin was also marketed. The single patch delivers 5 mg of testosterone per day. The incidence of itching at the application site was 12%, and 3% of users experienced erythema.
Other Testosterone Formulations
All of the testosterone replacement therapies discussed above are currently available in the United States; there are several other formulations that are now being investigated. Testosterone has been complexed with 2-hydroxypropyl-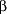 -cyclodextrin, to be administered sublingually at a dosage of either 2.5 or 5.0 mg 3 to 4 times a day.29 Orally administered testosterone undecanoate, which is available in Canada and Europe, is usually taken at a dosage of 40 to 80 mg, 2 to 4 times per day.30 Testosterone pellets are currently in use in the United Kingdom and in Australia; 3 to 6 testosterone pellets, 200 mg each, are implanted subcutaneously every 4 to 6 months.31 Testosterone buciclate, an experimental formulation, is a long-acting 17 -cyclodextrin, to be administered sublingually at a dosage of either 2.5 or 5.0 mg 3 to 4 times a day.29 Orally administered testosterone undecanoate, which is available in Canada and Europe, is usually taken at a dosage of 40 to 80 mg, 2 to 4 times per day.30 Testosterone pellets are currently in use in the United Kingdom and in Australia; 3 to 6 testosterone pellets, 200 mg each, are implanted subcutaneously every 4 to 6 months.31 Testosterone buciclate, an experimental formulation, is a long-acting 17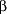 -hydroxyl ester of testosterone administered intramuscularly at a dosage of 600 mg every 12 weeks.32 -hydroxyl ester of testosterone administered intramuscularly at a dosage of 600 mg every 12 weeks.32
ADVERSE EFFECTS OF ANDROGEN REPLACEMENT
For most patients, androgen replacement therapy with testosterone is a safe, effective treatment for testosterone deficiency. Acne, weight gain, and edema may occur in patients with underlying edematous states such as congestive heart failure, hepatic cirrhosis, and nephrotic syndrome.1 Other adverse effects include excessive stimulation of libido, priapism, polycythemia, obstructive sleep apnea, urinary obstruction, and gynecomastia.1, 17, 32 Androgen replacement therapy stimulates erythropoiesis, occasionally resulting in polycythemia that may require reduction in the dose of testosterone, or even phlebotomy.1, 19, 33 If it is administered to prepubertal boys, premature closure of the epiphyses may occur.34 Administration of testosterone esters to some patients results in marked variations in serum testosterone levels that may be associated with emotional lability.35 The resulting changes in mood, libido, and sexual function may adversely affect the patient's sexual partner, especially when the couple has a long-established relationship.
The theoretical relationship between androgens and both benign prostatic hypertrophy and prostate cancer has been reviewed previously.36-40 Observations of hypogonadal men undergoing hormone replacement therapy and age-matched controls indicate that prostate volume and prostate-specific antigen levels are stimulated from the depressed level associated with the hypogonadal state to levels comparable with those of age-matched normal men.41 Before beginning androgen treatment, and yearly thereafter, the possibility of prostate carcinoma should be evaluated by digital rectal examination and a serum prostate-specific antigen level. Male breast cancer and known or suspected prostate carcinoma are contraindications to androgen replacement therapy.1
SUMMARY
Male hypogonadism, with many causes and a broad range of clinical manifestations, is underdiagnosed. Various types of androgen replacement therapy are available and new formulations, representing increasingly closer approximations of the ideal therapy, are under investigation. Until recently, intramuscular depot injections offered the most satisfactory combination of safety and efficacy, despite the fluctuations in serum testosterone levels that cause changes in sexual function, energy, and mood in some men. Because of limited effectiveness and a poor safety profile, currently available oral androgens are not recommended for replacement therapy. The scrotal patch delivers testosterone at a controlled rate but has been associated with elevated levels of DHT and altered testosterone-DHT ratios. The nonscrotal transdermal delivery system achieves normal diurnal levels of testosterone, normal levels of DHT and E2, and normal ratios of DHT testosterone and E2 testosterone. Local skin reactions are the most common adverse effect. Transdermal delivery systems permit the patient to self-administer medication, and to select among several anatomical sites for system placement. For these reasons, transdermal delivery represents a useful step toward the ideal androgen replacement therapy.
AUTHOR INFORMATION
Accepted for publication May 28, 1998.
Educational support for this project was provided by a grant from SmithKline Beecham Pharmaceuticals, Philadelphia, Pa.
Dr Winters is a consultant to SmithKline Beecham Pharmaceuticals.
Corresponding author: Stephen J. Winters, MD, University of Pittsburgh Medical Center/Montefiore 919N, 200 Lothrop St, Pittsburgh, PA 15213 (e-mail: winters{at}med1.dept-med.pitt.edu).
From the Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, Pa.
REFERENCES
 |  |
1. Matsumoto AM. Hormonal therapy of male hypogonadism. Endocrinol Metab Clin North Am. 1994;23:857-875.
ISI
| PUBMED
2. Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1-28.
FREE FULL TEXT
3. Resko JA, Eik-Nes KB. Diurnal testosterone levels in peripheral plasma of human male subjects. J Clin Endocrinol Metab. 1966;26:573-576.
FREE FULL TEXT
4. Mazer NA, Sanders SW, Ebert CD, Meikle AW. Mimicking the circadian pattern of testosterone and metabolite levels with an enhanced transdermal delivery system. In: Gurny R, Junginger HE, Peppas NA, eds. Pulsatile Drug Delivery: Current Applications and Future Trends. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft mbH; 1993:73-97.
5. Klinefelter HF. Klinefelter's syndrome: historical background and development. South Med J. 1986;79:1089-1092.
ISI
| PUBMED
6. Schwarz SD, Root AW. The Klinefelter's syndrome of testicular dysgenes. Endocrinol Metab Clin North Am. 1991;20:153-163.
ISI
| PUBMED
7. Hamerton JL, Canning N, Ray M, Smith S. A cytogenetic survey of 14,069 newborn infants. Clin Genet. 1975;8:223-243.
ISI
| PUBMED
8. Plymate S. Hypogonadism. Endocrinol Metab Clin North Am. 1994;23:749-772.
ISI
| PUBMED
9. Belanger A, Candas B, Dupont A, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086-1090.
ABSTRACT
10. Winters SJ. Endocrine evaluation of testicular function. Endocrinol Metab Clin North Am. 1994;23:709-723.
ISI
| PUBMED
11. Jackson JA, Kleerekoper M. Osteoporosis in men: diagnosis, pathophysiology and prevention. Medicine. 1990;69:137-152.
PUBMED
12. Behre HM, Kleisch S, Leifke E, Link TM, Neischlag E. Long-term effect of testosterone therapy in bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386-2390.
FREE FULL TEXT
13. Carrier S, Zvara P, Lue TF. Erectile dysfunction. Endocrinol Metab Clin North Am. 1994;23:773-782.
ISI
| PUBMED
14. Clopper RR, Voorhess ML, MacGillivray MH, Lee PA, Mills B. Psychosexual behavior in hypopituitary men: a controlled comparison of gonadotropin and testosterone replacement. Psychoneuroendocrinology. 1993;18:149-161.
FULL TEXT
|
ISI
| PUBMED
15. Davidson JM, Camargo CA, Smith ER. Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab. 1979;48:955-958.
FREE FULL TEXT
16. Skakkebaek NE, Bancroft J, Davidson DW, Warner P. Androgen replacement with oral testosterone undecanoate in hypogonadal men: a double blind controlled study. Clin Endocrinol. 1981;14:49-61.
PUBMED
17. Dobs AS, Hoover DR, Chen M-C, Allen R. Pharmacokinetic characteristics, efficacy, and safety of buccal testosterone in hypogonadal males: a pilot study. J Clin Endocrinol Metab. 1998;83:33-39.
FREE FULL TEXT
18. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
FREE FULL TEXT
19. Wang C, Swerdloff RS. Androgen replacement therapy. In: Bardin CW, ed., Current Therapy in Endocrinology and Metabolism. St Louis, Mo: CV Mosby; 1997:331-337.
20. Morales A, Johnston B, Heaton JWP, Clark A. Oral androgens in the treatment of hypogonadal impotent men. J Urol. 1994;152:1115-1118.
ISI
| PUBMED
21. Thompson PD, Cullinane EM, Sady SP, et al. Contrasting effects of testosterone and stanozolol on serum lipoprotein levels. JAMA. 1989;261:1165-1168.
FREE FULL TEXT
22. Physicians' Desk Reference. 50th ed. Montvale, NJ: Medical Economics; 1996:486-488, 1263, 2614.
23. Bals-Pratsch M, Knuth UA, Yoon Y-D, Nieschlag E. Transdermal testosterone substitution therapy for male hypogonadism. Lancet. 1986;2:943-946.
FULL TEXT
| PUBMED
24. Bals-Pratsch M, Langer K, Place VA, Nieschlag E. Substitution therapy of hypogonadal men with transdermal testosterone over one year. Acta Endocrinol. 1988;118:7-13.
25. Meikle AW, Mazer NA, Moellmer JF, et al. Enhanced transdermal delivery of testosterone across nonscrotal skin produces physiological concentrations of testosterone and its metabolites in hypogonadal men. J Clin Endocrinol Metab. 1992;74:623-628.
ABSTRACT
26. Meikle AW, Arver S, Dobs AS, Sanders SW, Mazer NA. Androderm: a permeation-enhanced non-scrotal testosterone trandermal system for the treatment of male hypogonadism. In: Bhasin S, Gabelnick HL, Spieler JM, Swerdloff RS, Wang C, eds. Pharmacology, Biology, and Clinical Applications of Androgens. New York, NY: Wiley-Liss; 1996:449-457.
27. Arver S, Dobs AS, Meikle AW, Allen RP, Sanders SW, Mazer NA. Improvement of sexual function in testosterone deficient men treated for 1 year with a permeation enhanced testosterone transdermal system. J Urol. 1996;155:1604-1608.
FULL TEXT
|
ISI
| PUBMED
28. Wilson DE, Kaidbey K, Boike SC, Jorkasky DK. Use of topical corticosteroid pretreatment to reduce the incidence and severity of skin reactions associated with testosterone transdermal therapy. Clin Ther. 1998;20:299-306.
FULL TEXT
|
ISI
| PUBMED
29. Stuenkel CA, Dudley RE, Yen SSC. Sublingual administration of testosterone-hydroxypropyl-cyclodextrin inclusion complex simulates episodic androgen release in hypogonadal men. J Clin Endocrinol Metabol. 1991;72:1054-1059.
FREE FULL TEXT
30. Gooren LJG. A ten-year safety study of the oral androgen testosterone undecanoate. J Androl. 1994;15:212-215.
FREE FULL TEXT
31. Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab. 1990;71:216-222.
FREE FULL TEXT
32. Behre HM, Nieschlag E. Testosterone buciclate (20 Aet-1) in hypogonadal men: pharmacokinetics and pharmacodynamics of the new long-acting androgen ester. J Clin Endocrinol Metab. 1992;75:1204-1210.
ABSTRACT
33. Krauss DJ, Taub HA, Lantinga LJ, Dunsky MH, Kelly CM. Risks of blood volume changes in hypogonadal men treated with testosterone enanthate for erectile impotence. J Urol. 1991;146:1566-1570.
ISI
| PUBMED
34. Richman RA, Kirsch LH. Testosterone treatment in adolescent boys with constitutional delay in growth and development. N Engl J Med. 1988;319:1563-1567.
ABSTRACT
35. Bardin CW, Swerdloff RS, Santen RJ. Androgens: risks and benefits. J Clin Endocrinol Metab. 1991;73:4-7.
FREE FULL TEXT
36. Horton R. Benign prostatic hyperplasia: new insights. J Clin Endocrinol Metab. 1992;74:504A-504C.
FULL TEXT
37. Partin AW, Oesterling JE, Epstein JI, Horton R, Walsh PC. Influence of age and endrocrine factors on the volume of benign prostatic hyperplasia. J Urol. 1991;145:405-409.
ISI
| PUBMED
38. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118-1126.
FREE FULL TEXT
39. Davies P, Eaton CL. Regulation of prostate growth. J Endocrinol. 1991;131:5-17.
FREE FULL TEXT
40. Brendler CB. The current role of hormonal therapy in the clinical treatment of prostatic cancer. Semin Urol. 1988;6:269-278.
PUBMED
41. Behre HM. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol. 1994;40:341-349.
PUBMED
RELATED ARTICLE
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 1999;8(3):207-209.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Impact of IGF-I and CYP19 Gene Polymorphisms on the Survival of Patients With Metastatic Prostate Cancer
Tsuchiya et al.
JCO 2006;24:1982-1989.
ABSTRACT
| FULL TEXT
Population variation in age-related decline in male salivary testosterone
Ellison et al.
Hum Reprod 2002;17:3251-3253.
ABSTRACT
| FULL TEXT
Andropause: Knowledge and Perceptions Among the General Public and Health Care Professionals
Anderson et al.
Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2002;57:M793-796.
ABSTRACT
| FULL TEXT
Testosterone in Aging Men
Applegate and Winters
Arch Fam Med 2000;9:221-221.
FULL TEXT
Testosterone reduces neuronal secretion of Alzheimer's beta -amyloid peptides
Gouras et al.
Proc. Natl. Acad. Sci. USA 2000;97:1202-1205.
ABSTRACT
| FULL TEXT
|