The Calcineurin-NFAT Pathway and Bone: Intriguing New Findings
The transcription factor nuclear factor of activated T cells (NFAT), first shown to be important in the T cell, is now recognized as having ever-wider roles in cell and tissue function. NFAT proteins are initially expressed as latent transcription factors present in a hyperphosphorylated form in the cytoplasm of resting cells. Dephosphorylation of critical regulatory amino-acid residues in NFAT by the protein phosphatase calcineurin unmasks conserved nuclear localization sequences and allows NFAT to enter the nucleus and, in concert with binding partners, such as members of the AP-1 family of transcription factors, activate gene expression (1). In T cells, genes whose transcription is activated by NFATc include a number of immunologically important factors, including interleukins 2, 3, 4, and 5, granulocyte/macrophage-colony stimulating factor, and tumor necrosis factor–α (2–7). Recent work has led to the recognition that the calcineurin-NFAT pathway affects other cells including heart, vasculature, skeletal muscle, and chondrocytes (8–14). The immunosuppressants cyclosporine A and tacrolimus (FK506) inhibit calcineurin and prevent the dephosphorylation and nuclear translocation of NFAT (15).
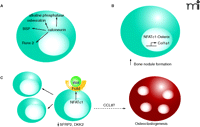
Recent studies by Koga et al. (16) and Winslow et al. (17) indicate that NFAT plays a critical role in osteoblasts, promoting their proliferation and differentiation, and leading to the synthesis of collagen and non-collagenous proteins. Earlier studies with cyclosporine A had shown that the agent decreased proliferation of both mouse MC3T3-E1 (18) and rat ROS 17/2.8 cells (19). Direct effects of calcineurin activity on osteoblast differentiation were recently examined by Sun and colleagues (20). The investigators first demonstrated that all isoforms of both the catalytic A subunit and the regulatory B subunit of this serine-threonine protein phosphatase were expressed in osteoblasts. They then introduced calcineurin into MC3R3-E1 osteoblastic cells as a TAT-calcineurin Aα fusion protein (to allow the transduction of the full length protein). Expression of the fusion protein directed the enhanced expression of Runx-2 (a transcription factor critical for commitment to an osteogenic lineage) (21), and the bone differentiation markers alkaline phosphatase, bone sialoprotein, and osteocalcin. Bone formation was increased in cultures of calvaria (i.e., the skullcap) from transgenic mice expressing the TAT-calcineurin Aα fusion construct. Conversely, calcineurin Aα−/− mice displayed osteoporosis and reduced mineral apposition.
Two recent studies have examined effects of NFAT per se in osteoblasts. Koga et al. (16) found that overexpression of NFATc1 in osteoblast precursor cells obtained from calvaria stimulated the activation of the type I collagen gene promoter (Col1a1). Correspondingly, bone nodule formation, an indicator of further osteoblast differentiation, was impaired in calvarial osteoblasts treated with the calcineurin inhibitor FK506 and in embryonic fibroblast cells from nfatc1−/− mice. To identify the mechanism driving this effect, the investigators determined the consequence of overexpressing NFAT on the transcriptional activities of three established osteoblast differentiation factors: Osterix, Runx2, and Smad. Osterix is a zinc-finger transcription factor involved in osteoblast differentiation and bone formation (22), and Smad proteins are signaling intermediates in the actions of bone morphogenetic protein-2, a stimulator of bone formation involved in bone morphogenetic protein-2 action (23). Koga et al. (16) found evidence of cooperative function––activation of the Col1a1 promoter––by the NFAT-Osterix combination only. Further studies showed that NFATc1 formed a complex with Osterix that was dependent upon calcineurin activation. Moreover, NFATc1 enhanced Osterix-dependent transcription via direct protein-protein interaction and not by direct binding to DNA. In fact, the recruitment of NFATc1 to the Col1a1 promoter in vivo was strictly dependent upon the presence of Osterix. Exactly how the interaction of NFATc1 with Osterix enhances transcription remains to be elucidated.
In the most recent study, Winslow et al. (17) generated mice that express a constitutively nucleus-localized NFATc1 variant, NFATc1nuc. Under the control of a tetracycline transactivator, the variant was expressed in thymus, splenic lymphocytes, and bone. Expression of the variant in bone cells was exclusively in osteoblasts, and the bone phenotype was characterized by an increase in osteoblast number and markedly increased bone volume. Histologic analysis revealed that the bone in the mutant mice had a less organized appearance than normal bone tissue, suggesting rapid osteogenesis. Serum osteocalcin and serum alkaline phosphatase, markers of osteoblast activity, were more than doubled. The increased bone mass was an early event, observed at embryonic day 16.5 (E16.5). Investigation of the possible mechanisms showed that the expressions of Wnt4 and Frizzled 9, members of the Wnt family whose signals can regulate bone mass (24, 25) were increased, while the expressions of the Wnt inhibitors Dickkopf2 and secreted frizzled-related protein 2 (SFRP2) were decreased. Other factors known to be involved in osteoblast proliferation and differentiation, including the transcription factor Runx2, were unaffected in mice expressing the mutation, and there was no defect in mineralization. Comparison of the findings in the calcineurin and NFAT studies reveals a difference in the observed responses: Runx-2 expression was elevated with calcineurin treatment but not with NFAT. Whether this represents a calcineurin effect that is independent of NFATc1 or reflects other divergent aspects of the models remains to be investigated.
Findings from three recent studies on effects of the calcineurin-NFAT pathway in osteoblasts. A. Overexpression of calcineurin increases the expression of the bone differentiation markers alkaline phosphatase, osteocalcin, bone sialoprotein (BSP), and Runx-2 20). B. Expression of NFATc1 increases the gene expression from the collagen 1a1 promoter and also increases the number of bone nodules (i.e., NFATc1 expression promotes bone formation) (16). NFATc1 forms a complex with the transcription factor Osterix. C. When a nuclear NFATc1 mutant (NFATc1nuc) is expressed in mice, expression of the mutant in bone is observed solely in osteoblasts (17). There was concomitant osteoblast proliferation, increased expression of Wnt4 and Frizzled 9, and decreased secreted frizzled-related protein 2 (SFRP2) and the Wnt antagonist dickkopf2 (DKK2). The expression of chemokine CCL8 was increased and osteoclastogenesis—whereby the fusion of precursor cells (not shown) to form mature multinucleated osteoclasts—was stimulated. Blue cells represent osteoblasts; the red cell represent a mature osteoclast.
In addition to the effects on osteoblasts, the NFATc1nuc mutant mice in the Winslow study (17) were noted to have increased osteoclast number and increases in markers of osteoclast activity and bone resorption. However, there was no increased expression of receptor activator of NF-κB ligand (RANKL), a cytokine produced by osteoblasts that activates osteoclast differentiation from monocyte cell line precursors and promotes fusion and survival of osteoclasts (26) or its physiological antagonist osteoprotegerin (OPG). The study presented evidence for increased expression of several monocyte chemoattractants in the NFATc1nuc mutant mice, and NFATc1 was shown to increase the chemokine CCL8. The osteoclastogenic effect of CCL8 appeared to be an indirect effect, as the authors stated that the direct addition of CCL8 did not elicit osteoclastogenesis in an in vitro model.
Effects of the NFAT pathways on bone are of particular clinical interest because of bone side effects of cyclosporine. When cyclosporine was used clinically as an immunosuppressant to prevent tissue rejection after organ transplantation, the treatment was associated with bone loss (27, 28). These clinical findings of bone loss with cyclosporine treatment were paradoxical because early studies showed that cyclosporine inhibits the resorption elicited by multiple factors in organ cultures of fetal limb bones (29) and neonatal calvaria (30). Consistent with the organ culture results on cyclosporine, recent studies established that expression of NFATc1 in precursor cells led to their differentiation into osteoclasts (31, 32). The results from the Koga et al. and Winslow et al. studies indicating that NFATc1 expression leads to osteoblast differentiation (16, 17), and the findings of Sun et al. (20) showing that calcineurin expression promotes the expression of bone differentiation markers suggest that the effects of cyclosporine to interfere with bone anabolic responses by blocking NFAT activation in osteoblasts could override the effects of the drug to inhibit osteoclast differentiation, with the result being a net loss of bone mass. Appealing as this explanation is, it is unfortunately inconsistent with the observations that the bone loss associated with cyclosporine is associated with high bone turnover (27, 28). The in vivo situation is complicated by multiple factors, and the mechanism of the bone loss is still unclear.
The findings reported in these three studies (16, 17, 20) are thought provoking for several additional reasons. The studies identify osteoblast factors that are regulated by the calcineurin-NFATc pathway and that lead to anabolic responses. This raises the possibility of involvement of the NFAT pathway in the action of anabolic agents for the treatment of osteoporosis and the existence of potential therapeutic targets within the pathway that could be employed to elicit anabolic effects on bone. Also interesting is that the effects of NFATc1 leading to stimulation of osteoclastogenesis were independent of RANKL and likely to involve chemokines suggesting that there are unexplored mechanisms leading to activation of the osteoclastogenic process (17). Finally, it is conceptually intriguing that the same NFAT signaling pathway that promotes the differentiation of cells that build bone also stimulates differentiation of the osteoclasts, the cells that break down the bone matrix.
<
Acknowledgments
The author thanks Neil Clipstone (Department of Pharmacology, Stritch School of Medicine, Loyola University) for his reading of the manuscript and helpful suggestions.
- © American Society for Pharmacology and Experimental Theraputics 2006
References

Paula H. Stern, PhD, is a Professor in the Department of Molecular Pharmacology and Biological Chemistry at the Feinberg School of Medicine, Northwestern University. The research interests of her laboratory include bone cell biology and endocrine pharmacology. The maintenance of bone involves interactions between systemic hormones, local cytokines and growth factors, and physical forces. Disorders that interfere with, amplify, or mimic the effects of these factors can result in abnormal bone remodeling and elicit pathological conditions that result in increased susceptibility to fracture. Prof. Stern’s laboratory seeks to define the pathways that lead to the cellular responses to these factors, in order to identify new potential targets for therapy. E-mail: p-stern{at}northwestern.edu; fax 312 503-5349.