Nascent Transcripts
Conformationally Restricted Ligands for the Elusive GBH Receptor
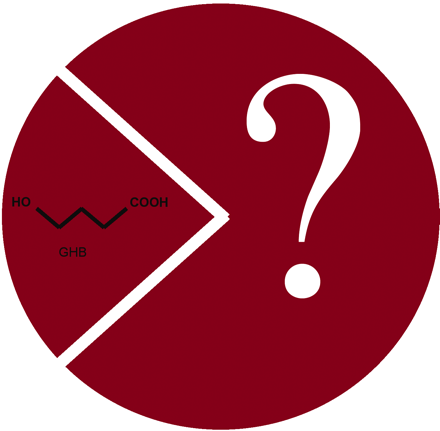
γ-Hydroxybutyrate (GHB) is an endogenous psychotropic compound thought to function as a neurotransmitter or neuromodulator in the mammalian brain. Although some of its effects may be mediated by GABAB receptors, the presence of high-affinity [3H]-GHB binding sites in the brain suggests the existence of a GBH receptor. In the October issue of J. Pharmacol. Exp. Ther., Wellendorph et al. report the development of novel, small-molecule, high-affinity, conformationally restricted ligands for this putative GHB receptor that have little affinity (IC50 > 1 mM) for GABA receptors. The best of these new GHB analogs has an affinity nearly forty-times that of GHB for high-affinity GHB binding sites, and several analogs inhibit the binding of [3H]-NCS-382 (a specific GHB binding site ligand) to high-affinity sites. These new ligands are powerful tools to probe the function of GHB and to characterize the physiological significance of the putative GBH receptor. [
] —DB Bylund, UNMC
Desensitized or Not Desensitized?: Morphine at the Mu-Opioid Receptor
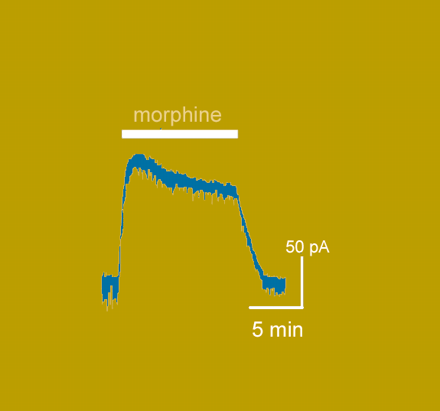
Morphine is an ancient and storied drug. Unfortunately, its therapeutic use is limited by tolerance. But what is the underlying mechanism of tolerance? The simple hypothesis that tolerance arises from receptor desensitization has been recently challenged. Instead—according to a new hypothesis—tolerance may reflect the failure of morphine-occupied receptor to undergo desensitization and endocytosis, which allegedly forces downstream intracellular signaling events to undergo compensatory regulation (such as increased cAMP production). In the October issue of Mol. Pharmacol., Dang and Williams reexamine the issue, using whole-cell recording from neurons in the locus ceruleus to measure morphine-induced K+ currents. The authors report that morphine-induced desensitization indeed occurs, albeit less pronounced than desensitization induced by [Met]5-enkephalin (ME), and that previous treatment of neurons with ME inhibits morphine-induced desensitization. Additionally, chronic application of morphine facilitates morphine-induced desensitization. The relevance of the observed receptor desensitization to tolerance thus remains an issue worthy of investigation. [
] —J Bockaert, CNRS, France
Inflammation and Infection Reduce Drug Metabolism
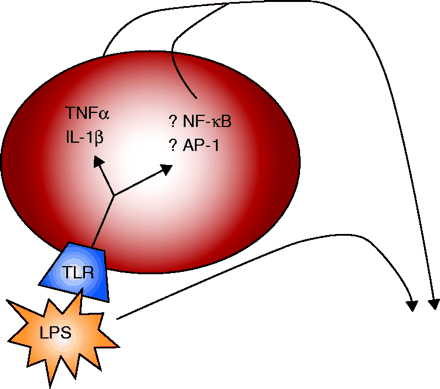
The cytochrome P450 enzyme system is essential for the efficient and predictable metabolism of many drugs. Cytokines released during inflammation and infection, however, can selectively decrease the expression of some members of the cytochrome P450 enzyme family, resulting in impaired drug metabolism and, potentially, drug-related toxicity. In the October issue of Drug Metab. Dispos., Abdulla et al. examine signal transduction pathways arising from inflammation of the brain, using an established model that is generated by injecting lipopolysaccharide directly into the CNS of rats. The authors demonstrate that several proinflammatory cytokines are produced in the liver as well as in the CNS. The results indicate that NF-κB and CCAAT-enhancer binding protein (C/EBP) participate in down-regulating the expression, respectively, of hepatic CYP2D5 and CYP2B1, two forms of cytochrome P450 that are important for xenobiotic metabolism. Understanding the effects of inflammatory and infectious diseases on drug metabolism may lead to strategies for improving drug efficacy and limiting adverse drug events. [
] —MO James, University of Florida
Uncovering Cloaked Opioid Receptor Function
Many G protein–coupled receptors are co-expressed in individual neurons, and the general expectation often is that expression equates to function. In the December issue of Mol. Pharmacol., Walwyn et al. demonstrate that although both the δ- and μ-opioid (DOP and MOP) receptors are expressed in murine dorsal root ganglion (DRG) neurons, only the MOP receptor is functionally coupled to Ca2+ channel regulation; however, this is not a static situation. DOP regulation of Ca2+ channels is “uncloaked” in MOP receptor knockout mice or by pharmacological inhibition of the MOP receptor. Furthermore, functional cloaking of the DOP receptor is restored by re-introduction of the MOP receptor into MOP-deficient DRG neurons. Although genetic removal or pharmacological masking of the MOP receptor results in upregulation of the DOP receptor, it remains unclear whether increased DOP levels alone result in receptor “uncloaking.” Nevertheless, these studies hint at strategies for targeting expressed but non–functional receptors to improve therapeutic indices or reduce side effect profiles. [
] —G Milligan, University of Glasgow, UK
- © American Society for Pharmacology and Experimental Theraputics 2005