Antimalarials
Shortages and Searches
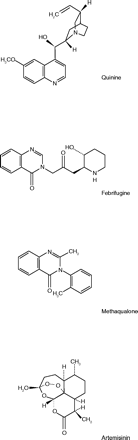
The course of drug discovery, like that of true love, does not run smooth. Neither does it tend to run in a straight line. The subject of these Reflections, the search for drugs to treat malaria, illustrates these statements. The urgent need for antimalarials during World War II spawned many projects among which was the discovery of an antimalarial alkaloid in a common ornamental bush. This substance did not survive clinical trials; however, an offshoot of the project led to a further compound that, although not an antimalarial, was a sedative-hypnotic. The latter drug was marketed, but fell victim to an undesirable form of popularity. Finally, we reflect briefly on a disastrous shortage of antimalarials, which exists even now, despite the passage of sixty years and enormous amounts of research effort.
World War II: The Quinine Pool
A few of us can recall, and the rest must try to imagine, the months immediately following Pearl Harbor. Japanese forces, facing little effective opposition, occupied the Philippines, Hong Kong, Malaya, Singapore, the Dutch East Indies, and Burma. One of the effects of Japan’s military success was that the United States was cut off from many strategic materials, including rubber and quinine.
The cinchona tree, the bark of which is the source of quinine, was first discovered in Peru and Ecuador, but by the 20th century, Latin America was a minor source and the main cinchona plantations were in the Dutch East Indies. By 1942, American armed forces were fighting in the South Pacific where they were exposed to malaria, but America’s supply of quinine had been shut off at the source. The situation was illustrated, for example, by an entry in a military nurse’s diary stating that eighty-five of the nurses evacuated from Bataan were suffering from malaria because there was no quinine (1).
Small amounts of quinine were scattered around the United States. Although there was essentially no malaria here, quinine had come into use for several other conditions. It was used to reduce fever in various febrile infections. Because of its intense bitterness, it was prescribed in “tonics” to stimulate appetite––a use that is reflected in its inclusion today in tonic water and bitter lemon. By virtue of its action on skeletal muscle, it was used to relieve nocturnal leg cramps. Accordingly, every pharmacy in the country kept a small bottle of bulk quinine or quinine sulfate for use in prescription compounding. Ruling that quinine could be used in civilian practice only for treatment of malaria, the War Production Board (WPB) in Washington “froze” the quinine on the drug store shelves. In the absence of civilian malaria, the opened bottles of quinine just sat there unused.
In September 1942, Ivor Griffith, president of the Philadelphia College of Pharmacy and Science, proposed to the WPB a plan whereby his college would solicit donations of quinine, its salts, and related alkaloids from its alumni. The WPB accepted his offer, but asked that the campaign at first be limited to Pennsylvania. Pharmacists and hospitals were solicited by all means of communication available. The college mailed special letters and bulletins; pharmaceutical salesmen talked up the drive; and the state pharmacists’ organization provided publicity. Donations soon poured in. Each gift was recorded and separate pools were made of each alkaloid and salt. These were sent to chemical manufacturing laboratories where they were processed and purified, and finally transmitted to the military. The pilot project collected and provided to the government, at no cost, about 1700 kg of quinine. The Quinine Pool then expanded to a nation-wide project under the sponsorship of the American Pharmaceutical Association, of which Ivor Griffith was the president in 1943–1944. Ultimately, nearly 7100 kg of quinine were donated to the American fighting forces (1).
The Cooperative Wartime Program
The government organized this nation-wide research program on an emergency basis. Chemical and pharmaceutical firms, colleges and universities, other private institutions, and individuals invested brainpower, manpower, and money. The federal government provided coordination and organization, as well as additional financial support, through the Office of Scientific Research and Development (OSRD). Later in the war, a Board for the Coordination of Medical Studies was formed, which advised and supervised the research done by the civilian institutions.
Compounds to be screened for antimalarial activity were accessed from many sources that included drug and chemical firms, and chemists in academic institutions who dealt directly with the program, or worked under OSRD contracts, or contributed through a commercial firm. Additionally, new series of compounds were synthesized; new methods of determining purity were developed, as were new methods of analysis. Advances were made in the biologic, pharmacologic, biochemical, immunologic, and clinical areas of malaria research. Important principles of chemotherapy were discovered, through which the use of quinacrine was improved and with which many new compounds were evaluated. The improved knowledge of how best to use quinacrine made quinine less important. Much of the stock collected in the Quinine Pool actually remained in the vaults unused.
The data generated in this vast wartime research program were summarized and published in 1946 in a three-volume work entitled A Survey of Antimalarial Drugs 1941–1945 (2). Recognizing that in perusing thousands of pages of research data the spirit animating the research may be lost, the editor of these volumes writes “The sympathetic reader will find in them something of the inspiration, patriotism, and cooperation of the men whose work they record” (3). Indeed, those connected with the program deemed it a major event in their lives, no matter how much they had achieved later. As a recent example, the obituary of a man who helped develop the drug Thorazine® in the 1950s, states “He earned a doctorate from the University of Virginia, where he researched antimalarial drugs during World War II” (4).
Hope From Hydrangea Leaves
Although the substances screened in the wartime program were mainly synthetic compounds, some botanicals were tested as well. Among those showing antimalarial activity were extracts of the dried roots of a Chinese herb known as Ch’ang Shan, or Dichroa febrifuga (5). In the 1940s, three alkaloids were isolated from this plant and named α-, β-, and γ-dichroine, respectively (6). Beginning in 1943, Koepfli et al. (7), working at the California Institute of Technology and Occidental College under the auspices of the Board for Coordination of Malaria Studies, with some support from K.K.Chen of Eli Lilly & Co., investigated the structures of these alkaloids. The most active alkaloid, which they named febrifugine, was identical to β-dichroine. Structurally, these alkaloids were 4-quinazolones, substituted at N-3.
Plant species of the same botanical family often produce similar secondary metabolites––alkaloids, glycosides, etc. Dichroa febrifuga belongs to the family Saxifragaceae, which includes currant, gooseberry, hydrangea, and syringa. A research team at Lederle Laboratories began testing related plant sources of the Saxifragaceae. They made the exciting observation of antimalarial activity in an acid aqueous extract of hydrangea leaves picked from their research director’s front lawn (8).
Following up this lead, the Lederle group screened numerous samples of the Easter variety of hydrangea, obtained from greenhouses. Certain cultivated hydrangea hybrids (greenhouse grown or semi-hardy garden grown) of unknown genetic make-up yielded significant amounts of a substance exhibiting antimalarial activity (9). Encouraged by the ready availability of this common garden plant, they initiated a high-priority program to isolate and characterize the active principle. In a series of papers [that began with reference (8)], the Lederle group reported the method of isolation and purification of the alkaloid febrifugine, its structure determination and synthesis, and the synthesis of analogues of the alkaloid. In addition, they confirmed that febrifugine obtained from hydrangea was identical to β-dichroine from D. febrifuga (8). A further bit of human interest in this history is the fact that the Lederle group’s botany advisor was Dr. Benjamin M. Duggar. Duggar is better known as the University of Wisconsin professor who was forced to retire at age seventy-one, then joined Lederle and discovered the first broad-spectrum antibiotic––Aureomycin® (chlortetracycline)––when he was seventy-five (8, 10).

Febrifugine must have been a most hopeful discovery at the time. First, it was present in a plant widely available in the US, and second, unlike quinine, it could be synthesized in commercial quantities. The toxicology and clinical testing, however, soon dampened the enthusiasm. Febrifugine’s anti-malarial activity was 64 to 100 times that of quinine, but its toxicity in animals was also correspondingly higher (11). Clinically, in a small study carried out on volunteer inmates at a federal prison in Texas, patients given 5 mg a day were unable to complete the treatment regimen because of severe nausea and vomiting. At 2.5 mg per day it was still emetic but well enough retained to produce temporary clearance of the parasitemia and fever. The authors concluded that its emetic action made the use of febrifugine impractical (11).
A similar result was published from a Mexican study in twenty-seven patients with acute malaria. Doses administered were 2 mg daily for five days or 0.099 mg/kg daily for fourteen days. The authors concluded that the alkaloid was not effective against Mexican strains of Plasmodium vivax and P. falciparum and that it had a marked emetic action (12). Thus febrifugine, despite the enormous amount of work done at Lederle, in California, and in China, failed to clear the clinical hurdle; however, an analog made it to the market in an unexpected way.
The Serendipitous Sleeping Pill
As mentioned above, the Lederle people had synthesized many analogues of febrifugine, but they were not the only ones. In India, Gujral et al. (13) prepared a series of twenty-two compounds with various substituted groups on N-3 of febrifugine. Testing in animals revealed that seven of these showed a significant hypnotic effect. The most potent was the tolyl derivative, which was somewhat superior to diallyl barbituric acid and considerably stronger than phenobarbital. In the 1950s, when this work was done, the barbiturates were important sedative-hypnotic drugs. This compound was developed as a sedative-hypnotic drug and assigned the generic name methaqualone.
The promising compound methaqualone was developed for marketing by William H. Rorer, Inc., and introduced in the U.S. in 1965. The etymology of Rorer’s trade name is interesting. Wishing to imply that the calming effects of the drug would produce a “quiet interlude”, they took the first two letters of “quiet” and the last syllable of “interlude”. In the middle they placed the double A of Maalox®, which was then Rorer’s most profitable product, and a household word. The trade name of methaqualone thus became Quaalude® (14).
Early clinical trials showed methaqualone to be effective as a mild sedative, tranquilizer, and hypnotic, and that it might be particularly useful for geriatric patients (15). It appeared to cause little or no hangover, an advantage over the barbiturates. Quaalude® quickly became popular, prompting several other firms to market methaqualone under their own trade names.

It would be a useful research project, though beyond the scope of these Reflections, to determine how and why methaqualone soon became an object of drug abuse. By 1972, reports of widespread street use were appearing in the press, and the expression “Luding out” had entered the language (16). It was especially popular among students in colleges and universities across the country. Counterfeit tablets came into circulation, and Rorer added a secret inactive ingredient to its tablets, to help distinguish the real from the counterfeit. The Drug Enforcement Agency (DEA) classified methaqualone as a Schedule II controlled substance, the most stringent level of control for drugs highly subject to abuse.
Fueling the abuse epidemic was the belief that Quaalude® was an aphrodisiac. The origin of this idea is unknown, but the persuasion could not be countered by science, or by the logical proposition that sex is best when one is awake. Obviously, drug abuse is not evidence-based; rumor is enough. A Philadelphia psychiatrist reported, for example, that some of her patients would describe loss of motor and intellectual control after taking Quaalude®, but would insist that the drug had no sedative effect on them (17).
The street popularity of Quaalude® had a chilling effect on its use for FDA-approved indications. Any physician who prescribed it might soon be besieged by “patients” seeking prescriptions for recreational use. That physician would also excite unwelcome attention from DEA. In this way, what was probably a reasonably good sleeping pill for short-term use lost its legitimate market during what has to be called an “unquiet interlude.”
The More Things Change.…
World War II ended sixty years ago, yet there are still problems with contemporary antimalarials reminiscent of the problems associated with antimalarial drugs of the 1940s. Currently, the shortage is worst in sub-Saharan Africa. According to Arrow et al. (18) chloroquine, the inexpensive mainstay of therapy for several decades, is rapidly losing its effectiveness because of resistant strains of Plasmodia. The treatment of choice now is the natural product artemisinin (and its derivatives), in combination with a synthetic antimalarial. The problems with this therapy are cost and supply. Artemisinin comes from the traditional Chinese medicinal herb Artemisia annua. Farmers in China and Vietnam grow the plant, and the active principle must be extracted and purified, making the cost of artemisinin’s production at least ten times that of chloroquine’s. The parallels are ironic. Like the cinchona tree, the plant source of artemisinin must be obtained from East Asia. There is also no commercially feasible synthesis for artemisinin, as there is none for quinine. In essence, a crisis again exists, though its geography has shifted and the drugs in short supply have changed.

In the United States malaria is rare, but a traveler or an American soldier returning from abroad may present with a fulminant attack of malaria requiring immediate parenteral treatment. The appropriate drugs are intravenous (iv) forms of quinine or artemisinin. Neither of these is FDA-approved or available in the United States. The closest substitute is iv quinidine gluconate, which is effective but has disadvantages. Malaria is an off-label use for this drug, cardiac monitoring of the patient is required, and the drug is not on the open market. Its manufacturer, Eli Lilly, maintains a supply and ships it rapidly for emergency use (19). All in all, a makeshift option which one would not expect to be necessary in this country.
The story of the search for quinine and quinine replacements during the forties, and of the methaqualone side-issue emerging from that search, illustrates the twists and turns inherent in drug discovery research. Perhaps an equally important lesson lies in American patriotism, which fueled research during World War II, and American concern for the less fortunate, which sparks the current drive to obtain adequate supplies of antimalarial medicines for Africa.
- © American Society for Pharmacology and Experimental Theraputics 2005
References
Benjamin M. Duggar


Stanley Scheindlin, DSc, RPh, holds graduate degrees in pharmaceutical chemistry and worked in drug product development and regulatory affairs. Now retired, he is a part-time consultant and writes freelance articles for pharmacy-related specialty publications.






