Neuropeptide S: A New Player in the Modulation of Arousal and Anxiety
Abstract
Neuropeptide S (NPS) is a newly identified transmitter that modulates arousal and fear responses. NPS activates an orphan G protein–coupled receptor that is expressed throughout the central nervous system, including brain centers that regulate sleep/wakefulness and anxiety. In contrast, the NPS precursor mRNA is found only in a few discrete nuclei in the brainstem as well as in a few scattered cells in the hypothalamus and amygdala. The most prominent expression of NPS precursor is found in a previously uncharacterized cluster of neurons in the pontine area, located between the noradrenergic locus ceruleus and Barrington’s nucleus. Central administration of NPS induces long-lasting arousal and suppresses all stages of sleep. In addition, NPS produces an anxiolytic profile in a variety of behavioral models. The unique pharmacological spectrum of NPS makes it an interesting target for pharmaceutical development. It also enhances our understanding of the neurobiological mechanisms of sleep/wakefulness regulation and the neuronal processing of stress.
Introduction
In order to understand behavior, we have to study the cellular and molecular events of neuronal communication. Neuronal communication relies on the stimulus-dependent release of diffusible neurotransmitters that activate specific receptors on adjacent neurons. The identification of the complete transmitter spectrum of the mammalian brain is the first and most important step on the way to elucidating higher brain functions and states of disease caused by dysregulation of such systems. The G protein–coupled receptors (GPCRs) are a superfamily of receptors that are activated by ligands of very diverse chemical classes, such as amines, peptides, protein hormones, amino acids, lipids, and nucleotides, as well as volatile odorants, photons, and inorganic ions such as calcium (1). The importance of GPCRs is evidenced by the fact that they regulate many peripheral and most brain functions. The human genome contains about 1000 genes for GPCRs, of which some 700 are olfactory and taste receptors. Of the remaining 300, about 160 have been matched to known natural ligands, leaving about 140—so-called “orphan” GPCRs—whose ligands remain to be identified. Identification of the natural ligands for these receptors is, of course, an essential prerequisite to studying basic neuronal pathways and other cell-signaling functions under normal and pathological conditions (2).
The discovery of novel neurotransmitters has important implications for both basic neuroscience and pharmaceutical development (3). Pharmaceutically, the discovery of a novel transmitter opens the opportunity for developing drugs with new therapeutic indications or mechanisms of action. Most importantly, the novel transmitter and its receptor offer an immediate tool for drug screening to identify small synthetic molecules that mimic or block the activity of the endogenous ligand.
Neuronal Systems Modulating Arousal and Stress
The brain contains multiple neuronal systems that evoke cessation of sleep states in response to sensory stimuli and maintain wakefulness during the active part of the day (4). Anatomically, these brain structures are located in the brainstem, thalamus, posterior hypothalamus, and basal forebrain. As an integrated system, they send ascending stimulatory projections to the cortex and descending projections, via the spinal chord, that elevate muscle tone and sensory-motor responsiveness. Once awake, maintenance of arousal and attention is critical for effective processing of sensory information and cognitive processes. It is believed that the noradrenergic locus ceruleus (LC) in the brainstem plays an important role in promoting such attentiveness. During the natural sleep-wake cycle, LC neurons show rhythmic firing during wakefulness, decreased firing during slow-wave sleep, and are virtually silent during paradoxical sleep (i.e., REM sleep) (5). Maximal discharge of LC neurons is observed during highly aroused conditions such as stress (6). Electrical and chemical activation of the LC produces cortical activation, and drugs that enhance noradrenaline release cause cortical activation accompanied by behavioral arousal (7). Increased noradrenergic neurotransmission is also associated with elevated anxiety-like behavior (8).
The dorsolateral pontine tegmental region is one of the areas that have been strongly implicated in both sleep regulation and stress-related behaviors. The dorsolateral tegmental region contains several distinct nuclei, such as Barrington’s nucleus and the LC, and also comprises unidentified neurons, outside of the LC proper, such as the peri-LC region (9). The LC is a major source of noradrenergic input to the cortex and an important regulator of arousal and anxiety (10). Barrington’s nucleus, the pontine micturition reflex center, expresses corticotrophin-releasing factor (CRF) as its peptidergic neurotransmitter (11). Besides its established role in bladder control, Barrington’s nucleus has been found to participate in the neuronal cascade activated by stress (12).
Hypocretins/orexins (Hcrt/Ox) are two closely related peptides that were initially identified as ligands of two orphan GPCRs (13). In addition to an effect on energy homeostasis, they were later found to be critically involved in the maintenance of wakefulness (14). Human narcoleptic patients are deficient in Hcrt/Ox-producing neurons (15), and two animal models (i.e., narcoleptic dogs and prepro-Hcrt/Ox knockout mice [16, 17]) confirm the hypothesis that Hcrt/Ox is necessary to maintain a state of stable wakefulness. Anatomically , Hcrt/Ox-producing neurons appear to innervate arousal centers in the brainstem, such as the histaminergic tuberomamillary nucleus, the noradrenergic LC, and serotoninergic neurons of the dorsal raphe nucleus (18–20).
In addition to the known neurotransmitters and neurocircuits that are involved in arousal and anxiety, there could be other important regulators and structures in the CNS that have not yet been uncovered. Although the important regulatory role of the noradrenegic LC system in modulation of sleep-wake states and stress has been extensively documented, several studies have found no major disruption of EEG activity after selective cytotoxic lesions of tyrosine hydroxylase–(TH)-positive LC neurons or genetic ablation of the noradrenaline-synthesizing enzyme dopamine beta-hydroxylase (21, 22), indicating that additional mechanisms might exist. Our present data indicate that the novel neuropeptide, which we call neuropeptide S (NPS), is expressed in a previously undescribed group of cells located directly adjacent to the LC proper. In addition, our functional studies reveal that NPS could be a novel arousal-promoting system (23). Interestingly, the close vicinity of NPS-producing neurons and the noradrenergic neuronal cluster in the LC indicate that this brainstem area might contain two independent transmitter systems that regulate arousal and anxiety behavior.
Structure, Distribution, and Pharmacology of Neuropeptide S
Database searches of genome sequences in GenBank (finished and raw data) reveal that the primary structure of NPS is highly conserved among vertebrates. Curiously, the peptide is absent in fish genomes (e.g., zebrafish and fugu) and is also not found in amphibian or reptile DNA sequences, indicating that this transmitter arose relatively late during vertebrate evolution. As shown in Figure 1⇓, the N-terminal residue of NPS in all species analyzed is always serine, which prompted our use of the moniker NPS. As with other neuropeptides, NPS is cleaved from a larger precursor protein containing a hydrophobic signal peptide and proteolytic processing sites (23). The NPS receptor is a typical GPCR, containing seven membrane-spanning domains. The NPS receptor shows moderate homology to other members of the GPCR family, the closest relatives being the vasopressin (i.e., V1a and V2) receptors.
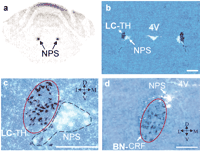
In situ hybridization reveals that the NPS receptor mRNA is widely expressed throughout the nervous system, with highest levels found in cortex, thalamus, hypothalamus, and amygdala, and low levels of expression in the brainstem. In contrast, the NPS precursor mRNA is expressed strongly in the LC area (Figure 2⇓), the principle 5 sensory nucleus, and the lateral parabrachial nucleus of the brainstem. Only a few scattered positive cells could be detected in other brain areas, such as the amygdala and hypothalamus.
A more detailed analysis reveals that the NPS-producing neurons in the LC area are directly adjacent to the TH-positive cells of the LC proper; NPS does not colocalize with CRF-producing neurons of Barrington’s nucleus (Figure 2⇓). From these data, we conclude that the NPS-expressing neurons lie caudally to Barrington’s nucleus and at the mid level of the LC. This unique anatomical pattern defines a previously unrecognized population of cells located between the noradrenergic LC proper and Barrington’s nucleus.
Cells stably expressing the NPS receptor protein display a transient increase in intracellular free Ca2+ concentration after activation of the receptor with nanomolar concentrations of NPS. A radiolabled analog of NPS binds the receptor with high affinity (Kd = 0.3 nM) (23). Together, these data indicate that NPS might behave as an excitatory transmitter in vivo by elevating intracellular Ca2+ concentration.
NPS Promotes Arousal and Anxiolytic-like Behavior
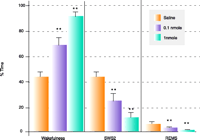
When injected centrally, NPS produces long-lasting arousal that is independent of novelty. In mice habituated to the test chamber, low doses (0.1 or 1 nmole) of NPS increase locomotor activity, lasting for almost one hour, whereas mice injected with vehicle display no change in their exploratory behavior (23). Because arousal is an important component of wakefulness, we also analyzed the effect of NPS on sleep patterns in rats during their normal period of inactivity. NPS can suppress all stages of sleep during the first hour post administration (Figure 3⇓), again indicating that NPS might be involved in the induction or maintenance of wakefulness.
The high level of NPS receptor expression in the amygdala, along with the close proximity of NPS-producing cells to the noradrenergic LC, led us to hypothesize that the NPS system might also be involved in modulation of emotional behavior. When we tested the effect of centrally administered NPS on anxiety-like behavior in various paradigms, we found that NPS can produce an anxiolytic-like profile that was not confounded by the motor-activating effects of the peptide (23). Intriguingly, NPS increases the time mice spend exploring the less protected or brighter areas of their test environments (e.g., open field, light-dark box, elevated plus maze). On the other hand, NPS administration reduces the time mice spend burying unfamiliar objects (i.e., marbles), demonstrating an overall anxiolytic profile for the peptide.
Comparison to Other Neurotransmitter Systems Involved in Arousal and Anxiety
Compared to other transmitters or drugs that have well-known effects on sleep and emotional behavior, the pharmacological identification of NPS as a potent modulator of arousal and stress is unique (Table 1⇓). For example, stimulants such as amphetamine and cocaine potently induce arousal but appear to be anxiogenic at the same time (24, 25). Central administration of Hcrt/Ox is able to suppress sleep and induce profound wakefulness (26, 27), but the peptide does not affect anxiety-like behavior (26). Similarly, the anti-narcolepsy drug modafinil, whose mechanism of action is still unknown, can increase wakefulness but does not modulate anxiety (28). Typical anxiolytic drugs such as the benzodiazepines do not affect locomotion at low anxiolytic doses but tend to inhibit motor activity at higher doses (29). These examples show that NPS produces a unique pattern of behavioral effects, and endogenous NPS might be involved in modulating sleep/wake states as well as neuronal responses to stress.
Conclusion
The discovery of NPS as an important regulator of arousal and anxiety sheds new light on the neurochemistry and biological basis of these complex brain functions. It also opens the opportunity to start developing synthetic molecules that can be evaluated as drugs modulating the function of this novel neurotransmitter system. NPS agonists might find clinical application in the treatment of hypersomnia or narcolepsy, and might well be useful to treat anxiety disorders without associated sedating side effects. On the other hand, NPS antagonists might be novel therapeutics for insomnia. It is hoped that such compounds will soon become available for research, because apart from any clinical application per se, they will greatly help to advance our understanding of the physiological functions of the NPS system.
Rainer K. Reinscheid, PhD, is an Assistant Adjunct Professor in the Department of Pharmacology at the University of California, Irvine. Send correspondence to RKR. E-mail rreinsch{at}uci.edu; fax 949-824-4855.
Yan-Ling Xu, MD/MS, is a graduate student in the laboratory of Olivier Civelli, PhD, who is the Eric L. and Lila D. Nelson Chair in Neuropharmacology at the University of California, Irvine.
Prototypic Drugs Modulating Arousal and/or Anxiety
Alignment of NPS peptide structures from various species. Sequences were deduced from GenBank finished and unfinished genome sequences. Amino acids different from human NPS are highlighted in red.
Localization of NPS precursor mRNA expression in the rat brainstem by in situ hybridization. A. Autoradiogram showing strong labeling of NPS-expressing cells in the LC area of the rat brainstem. B. Double in situ hybridization for NPS precursor (white) and tyrosine hydroxylase (TH, dark blue) demonstrates that the NPS-producing cells are not colocalized with noradrenergic LC neurons (LC-TH), but rather form a separate cluster in close vicinity. (4V, fourth ventricle.) C. Higher magnification of (B). The cluster of noradrenergic LC neurons (LC-TH) is circled with a solid red line; the NPS cluster is marked with a broken green line. D. Double in situ hybridization for NPS precursor (white) and corticotropin-releasing factor (CRF, dark blue). CRF-positive cells form the ovoid-shaped Barrington’s nucleus (BN-CRF; solid red line). Scale bar in B is 500 μm, and 250 μm in C and D. Crossed arrows denote orientation of slides: D, dorsal; V, ventral; L, lateral; M, medial. Photomicrographs are adapted from (23).
NPS modulates vigilance states. Effect of central administration of 0.1 and 1 nmole NPS or vehicle on vigilance states in rats during the first hour post injection. EEG and EMG recordings were scored for different stages of vigilance, indicating either wakefulness, slow wave sleep stage 2 (SWS2), or paradoxical sleep (REMS), and total time spent in each vigilance state was calculated. NPS (0.1 and 1 nmole) significantly increases wakefulness and reduces SWS2 and REMS. N=8 animals per group; ** significantly different from saline-injected animals, p < 0.01. [Note: Scores for SWS1 were omitted from the graph due to the small contribution of SWS1 to total sleep time, averaging less than 5%. NPS also reduced SWS1 significantly; see (23).]
Acknowledgments
This study was supported in part by grants from NIMH and NARSAD to R.K.R.
- © American Society for Pharmacology and Experimental Theraputics 2005