Ephedra: Once a Boon, Now a Bane
- Stanley Scheindlin, DSc, RPh, received his degrees in pharmaceutical chemistry and is retired after more than forty years in the industry. His work is published in research journals and in specialty publications in pharmacy.
Those who forget the past are not always fated to repeat it—sometimes the consequences are even worse. The problem of ephedra in the year 2003 arose largely because we have forgotten some excellent pharmacological research of eighty years ago.
The sudden death of Steve Bechler, a promising young pitcher for the Baltimore Orioles, during spring training in February 2003, was attributed to his use of an ephedra-containing dietary supplement. This focused attention on a long-simmering question: is ephedra safe, or should it be banned? The question was quickly taken up by sports columnists, thus assuring wide public awareness of the issue.
Although the Food and Drug Administration (FDA) already had reports of at least 100 deaths linked to the use of ephedra—not to mention nearly 17,000 nonfatal adverse events—the Agency took no urgent action. However, the FDA commissioned the RAND Corporation to review all studies and data on ephedra and to submit a report by Spring 2003. Spurred by Bechler’s death, the report was rapidly completed and Health and Human Services Secretary, Tommy Thompson, and FDA Commissioner, Mark McClellan, hurriedly released RAND’s findings at a special news conference on February 28, 2003 (1). On March 1, 2003, the news media reported that the FDA was not only issuing a proposal to require ephedra products to carry warning labels stating that they can cause heart attacks, strokes, even death, but also broaching the idea of a possible ban on some ephedra-containing products (1).
These media reports were misleading because of what they omitted. The FDA’s announcement in the Federal Register of March 5, 2003 was not a new proposal (2), but rather reopened the comment period of a proposal on ephedra first published on June 4, 1997. In fact, no final ruling has ever been published.
While the foregoing seems like an indictment of the FDA, the following must be considered in mitigation. Ephedra, an herb legally sold as a dietary supplement, is widely used to promote weight loss and to provide an energy boost and is reputed to be popular among athletes. The FDA is prevented from acting quickly by the Dietary Supplement Health and Education Act, which Congress passed in 1994. Under this law, dietary supplements, including herbs, may be sold without FDA review, and the FDA must prove them dangerous before it can have them removed from the market. The purveyors of ephedra products, said to enjoy sales of $3 billion a year, mounted a formidable campaign, claiming that fifty-five clinical studies support the safety of ephedra. Most likely it was this staunch position that caused the FDA to turn to RAND for an impartial evaluation.

Yet, was it necessary for the FDA to jump through hoops for six years and to pay for further meta-analyses and sophisticated statistical calculations? The actions of ephedra and its active constituents were clearly elucidated by the elegant pharmacological studies of K.K. Chen and Carl F. Schmidt. Their studies, reported between 1924 and 1930, lay outside the range of computerized databases, but still live in standard reference sources and in the memories of pharmacologists, pharmacists, and physicians who now are either retired or approaching retirement age.
Ephedra: The Herb
Ephedra consists of the stems and leaves of plants of several species of the genus Ephedra. Also known by its Chinese name, ma huang, ephedra has been used in traditional Chinese medicine as a stimulant, anti-asthmatic, and diaphoretic for over 5000 years. In America, settlers used it in colonial times for colds, kidney disorders, and venereal diseases. Ephedra has a number of common names, or synonyms, which are rather intriguing (3). One, for example, is “Teamster’s tea.” Teamsters would drink a tea made from ephedra to help them stay awake during long hauls. Another name, “whorehouse tea,” suggests that brothel patrons used it for a Viagra-like effect. The names “Mormon tea” and “Brigham tea” have the same implication, as a Mormon husband might have had to satisfy several wives.
Ephedrine: The Active Component of Ephedra
Ephedra contains as its active constituents ephedrine and several other alkaloids. Ephedrine was first isolated in 1887 by Nagajosi Nagai, a professor at Tokyo University. Although Japanese scientists reported in 1917 that ephedrine was a sympathomimetic (i.e., its actions were similar to those of adrenaline), its medicinal potential was overlooked in the West until the Chinese researcher, K.K. Chen and the American physician, Carl Schmidt, came on the scene.
Ku Kuei Chen received his PhD in biochemistry and physiology from the University of Wisconsin in 1923 and went back to teach pharmacology at Peking Union Medical College in Peking, China. Having an interest in Chinese herbal medicine, he began to study ma huang (Ephedra vulgaris). He enlisted the assistance of Carl F. Schmidt, a colleague at Peking Union, who had obtained his MD from the University of Pennsylvania (4).
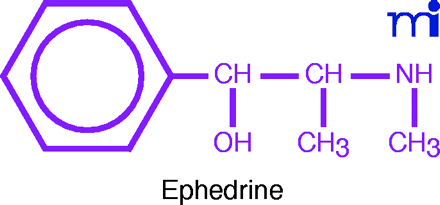
In their preliminary investigation, Chen and Schmidt administered an IV injection of an extract of the crude drug to an anesthetized dog. They noted a sustained rise in blood pressure, acceleration of the heart, and constriction of the kidney blood vessels. Seeking the active principle, Chen re-isolated ephedrine and recognized it as the alkaloid discovered by Nagai. Exhaustive studies were conducted on the pure alkaloid: the action of ephedrine was similar to, but weaker than that of adrenaline, and ephedrine showed a much longer duration of action. Unlike adrenaline, ephedrine was active by mouth as well as by injection. Chen and Schmidt reported their first studies in 1924 (5) and continued their exploration of the pharmacology of ephedrine and other ephedra alkaloids.
Recognizing the therapeutic potential of ephedrine, in view of its duration of action and effectiveness per os, Chen and Schmidt provided supplies of ephedrine for clinical investigation to T.G. Miller at the University of Pennsylvania and to L.G. Rountree at the Mayo Clinic. The clinical investigations, reported in the literature between 1925 and 1926, led to ephedrine’s approval by the Council of Pharmacy and Chemistry of the American Medical Association in 1926 (4). Recognition by the Council was the equivalent of FDA approval, because prior to 1938 there existed no mechanism for review of new drugs by the federal government.
In 1930, Chen and Schmidt published a monograph on the chemistry, pharmacology, and therapeutics of ephedrine and related alkaloids (6). Schmidt subsequently became Professor of Pharmacology at the University of Pennsylvania Medical School, and Chen joined Eli Lilly & Co., in Indianapolis, where he became a highly respected figure in new drug discovery.
Ephedrine as a Medicine
Chen and Schmidt’s monograph (6) discusses the routes of administration, dosage, and side effects of ephedrine, as well as the findings of numerous clinical trials. The therapeutic uses investigated included asthma, hay fever, bronchitis and emphysema, pertussis (to relieve coughing and whooping), spinal anesthesia, hypotension, shock, nasal congestion, mydriasis, urticaria, dysmenorrhea, and as an antidote for narcotic drugs.
From its initial acceptance by the AMA Council in 1926 to the 1950’s, ephedrine—as the base, hydrochloride, or sulfate—was one of the most widely used drugs in the pharmacopeia. Pharmacologically, it is an α - and β -adrenergic agonist. As a powerful central nervous system (CNS) stimulant, ephedrine was used for treating morphine or barbiturate overdosage, narcolepsy, and catalepsy. Its therapeutic cardiovascular (CV) uses were numerous; it was given by injection to bolster blood pressure during spinal anesthesia, and orally for chronic hypotensive states and postural hypotension.
The bronchodilator action of ephedrine made it useful in treating asthma, at a time when the only other available bronchodilator was theophylline. Additionally, ephedrine was used for the relief of various allergic disorders, and as a nasal decongestant in colds, sinusitis, and vasomotor rhinitis. Many combination products containing ephedrine were marketed as well (7, 8).
The side effects of ephedrine were well known and included headache, palpitations, sweating and thirst, giddiness, difficulty in urination, muscle weakness, tremors, restlessness, and insomnia (7). Still, when used in appropriate dosages, the benefits of the drug outweighed its adverse effects; like all medicines, ephedrine was useful within its limitations.
Ephedrine fell out of use because superior drugs were developed. As a nasal decongestant it was replaced by pseudoephedrine, which has weaker CNS and CV effects. Small amounts of pseudoephedrine, by the way, are found in ephedra. For bronchodilation, more selective β2-adrenergic agents (e.g., albuterol and salmeterol) were discovered. Numerous other sympathomimetic amines are now available for various CNS and CV needs. Over the past forty years, numerous drugs, able to replace ephedrine in several indications, have been marketed and strongly promoted. As a result, ephedrine has been forgotten by the public and even by most health professionals.
The Lessons of Ephedra
What lessons can we learn from ephedra? There are two lessons – one general and one specific. First, the problems caused by its widespread, unsupervised use are part of the general problem of dietary supplements, which continue to be promoted and sold without evidence of safety, without adequate standardization, and without reporting of adverse effects. The advertising is reminiscent of a century ago, when concoctions consisting largely of alcohol were hawked as cures for serious diseases of all kinds. In the past year two prestigious clinical journals have published three editorials calling for government regulation of dietary supplements (9 –11). These seem to have fallen on deaf ears as far as Congress, the media, or the public are concerned. Steve Bechler’s death has become yesterday’s news, and ephedrine’s status has not changed since the FDA’s flurry of activity in early March.
However, ephedra teaches a more specific lesson as well. Before it was “discovered” by the medicine men of the 1990’s, ephedra, and particularly its main alkaloid ephedrine, already had a long history, well documented in the pharmacological and medical literature. Calling ephedra an herb makes it sound benign. Actually, it is a potent botanical drug. Calling it a dietary supplement is even more misleading. To this non-lawyer, it appears clear that a review of the literature, starting with the work of Chen and Schmidt, would have given the FDA a solid basis for declaring ephedra to be a drug marketed without an approved new drug application. As such, it could have been removed from the market before 100 avoidable deaths and countless injuries occurred.
To forget or ignore research because it is eighty years old is done at our peril. This is the lasting message of the ephedra affair of 2003.
- © American Society for Pharmacology and Experimental Theraputics 2010