The TASK Family: Two-Pore Domain Background K+ Channels
- 1Departments of Pharmacology and
- 2Anesthesiology, School of Medicine, University of Virginia, Charlottesville, VA
- Address correspondence to DAB. Email: dab3y{at}virginia.edu; fax 434-982-3878.
Abstract
The recent identification of a distinct family of genes encoding two-pore domain potassium channels that generate background, or leak, K+ currents has permitted the analysis of the molecular underpinnings of these heretofore poorly understood currents. In the case of the TASK subfamily of channels, particularly TASK-1 (KCNK3) and TASK-3 (KCNK9), detailed characterization of cloned (and heterologously expressed) channels has revealed sets of unique properties that, together with information regarding sites of expression, have permitted identification of their native counterparts. This analysis has demonstrated that native TASK-like channels are subject to multiple forms of modulation (e.g., by protons, volatile anesthetics, oxygen tension, and receptor-mediated signaling pathways), which serve as a basis for participation in a number of physiological mechanisms. In this review, we describe the salient features of cloned TASK channels, present evidence for their functional expression in a number of tissues (e.g., brain, heart, and adrenal gland), and discuss possible roles for these channels in pH-dependent physiological processes (e.g., breathing and arousal) and in the clinical actions of inhalation anesthetics.
Introduction
Signaling in electrically excitable cells depends on the existence of a polarized membrane potential in which the cell interior is negative with respect to the exterior. Resting (or “leak”) K+ conductances are important in setting neuronal membrane potential. The relatively recent discovery, through molecular cloning, of a new family of K+ channels—the so-called KCNK (or K2P) channels—with properties expected of resting K+ channels and distinct from all previously identified K+ channel families now elucidates the molecular basis for these leak K+ channels.
Previous observations using native neuronal preparations presaged the existence of a distinct class of leak K+ channels: voltage-independent K+ -selective currents were revealed by the actions of neuromodulators and drugs. For example, a form of behavioral plasticity in the sea hare (Aplysia californica ) involves a mechanism whereby serotonin inhibits a background K+ channel—the so-called S channel—with properties unlike those of voltage-dependent K+ channels (1). A similar form of neurotransmitter-mediated inhibition of leak K+ currents is also prevalent throughout the mammalian CNS (2, 3). In addition, early studies documented the activation of invertebrate neuronal leak K+ channels by compounds such as arachidonic acid and volatile anesthetics (4, 5) , and these observations have been extended to mammalian systems (6–8). Importantly, various members of the newly identified KCNK channel family possess similar modulatory potential, identifying members of this family as neuronal leak K+ channels (9–12).
In this review, we describe briefly the characteristics of the KCNK channel family, in comparison to those of the other K+ channel families. We then focus on a particularly well-studied subgroup of KCNK channels, the so-called TASK (T WIK-related a cid-sensitive K+ ; TWIK, for t andem P domains in a w eak i nwardly rectifying K+ ) channels (e.g., KCNK3 and KCNK9), along with their suggested physiological and clinical roles. The reader is directed to a number of recent and excellent reviews that provide more general treatment of the entire KCNK gene family, as well as their possible functional roles in the brain and other tissues (9–12).
Protein Subunits of K+ Channels
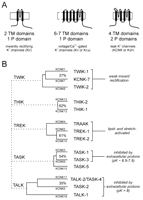
There are three major families of K+ channel proteins, each with distinct structural features and functional characteristics (Figure 1A⇓; reviewed in 9, 13–15). The voltage-dependent K+ channels (Kv), such as the classical delayed rectifier and transient voltage-dependent K+ channels, Ca2+ -dependent K+ channels, and hyperpolarization-activated, cyclic nucleotide-gated cationic (HCN) channels, arise from tetrameric associations of the first family of subunits, which have six (or sometimes seven) membrane-spanning domains; the voltage sensor comprises charged amino acids of the fourth transmembrane domain. The K+ -selective pore of Kv channels depends on the re-entrant helix (Figure 1A⇓, middle panel) between the fifth and sixth membrane-spanning regions that reside in each of the four channel subunits.
Potassium ion channel gene families. A. Three major K+ channel gene families have been identified: Voltage-gated (Kv) channels have six (or seven) transmembrane segments and one pore domain; inwardly rectifying (Kir) channels present two membrane spanning regions and one pore domain. These channels form as tetramers. The newly identified, weakly rectifying (KCNK) channels are predicted to have four transmembrane regions and two pore domains, each one resembling two Kir channels arranged in tandem. KCNK channels are believed to form as dimers. B. The fourteen known human two-pore-domain K+ channel genes are presented in a phylogenetic tree, and placed into five sub-groups as described (11, 12). Multiple naming schemes for these channels are in current use; however, only two are shown. The Human Genome Organization uses the KCNK designation preceding a number that reflects the order of discovery of each of the genes (as shown here). The International Union of Pharmacology (IUP) adopted a very similar scheme, but replaced the KCNK with a K2P prefix (not shown); the IUP group suggests that a different nomenclature linking clearly related channels will likely be forthcoming (97). One such alternative naming scheme that is in popular use employs a set of acronyms based on salient physiological or pharmacological properties (provided here). TWIK, tandem of P domains in a weak inwardly rectifying K+ channel; THIK, Tandem pore domain halothane-inhibited K+ channel; TREK, TWIK-related K+ channel gene; TRAAK, TWIK-related arachidonic acid-stimulated K+ channel; TASK; TWIK-related acid-sensitive K+ channel; TALK, TWIK-related alkaline pH activated K+ channel.
Members of the second family of K+ channel proteins associate into tetramers to give rise to the inwardly-rectifying K+ channels (Kir). Each of the four subunits possesses only two transmembrane domains, which flank the pore-forming loop domain (Figure 1A⇑, left panel). Some Kir channels show constitutive activity at resting membrane potentials, whereas the activity of other channels is influenced by modulators such as G proteins, nucleotides, polyamines, and physiological concentrations of protons (13, 16–20).
Members of the third family of K+ channel proteins, the KCNK proteins (9, 10) , are thought to dimerize. Each KCNK subunit contains four transmembrane segments and two pore-forming domains (Figure 1A⇑, right panel). In the genomes of C. elegans and D. melanogaster , this family comprises the largest number of putative K+ channel subunits (15, 21, 22) ; in mammals, fourteen subunits have been identified to date (see Figure 1B⇑), although only eleven are known to be functional. The KCNK channel proteins are distinctive in that they are generally constitutively active at resting membrane potentials and generate currents that rectify only very weakly. The KCNK proteins can be grouped according to sequence and functional similarities into five subfamilies: the weakly inwardly rectifying TWIK channels (TWIK-1, TWIK-2, and the nonfunctional KCNK7); the halothane-inhibited THIK-1 channel and related non-functional THIK-2; the arachidonic acid– and mechanosensitive TREK channels (TREK-1, TREK-2, and TRAAK); the alkaline-activated TALK channels (TASK-2, TALK-1, and TALK-2/TASK-4); and the acid-sensitive TASK channels (TASK-1, TASK-3, and the non-functional TASK-5). We will focus on TASK-1 and TASK-3.
Properties of Cloned TASK Channels
Electrophysiological Properties of Cloned TASK Channels
TASK-1 (KCNK3) and TASK-3 (KCNK9) are two-pore–domain channels that generate pH-sensitive whole-cell K+ currents with little time-dependence (or extremely fast kinetics) and weak rectification. In physiological asymmetric K+ solutions, this rectification can be accounted for entirely by the Goldman-Hodgkin-Katz (GHK) constant field equation, indicating that they function like leak K+ channels (23–29).
The unitary conductance of a TASK-1 channel (∼14 pS) is one-half that of a TASK-3 channel (∼28 pS), although macroscopic currents arising from these two TASK channels are very similar in terms of kinetics and voltage-dependence (23–28). At the single channel level, a slight inward rectification in unitary conductance is offset by a modest voltage-dependent increase in activity at depolarized potentials to coordinately account for the apparent open rectification of TASK channel–mediated whole-cell currents (24, 26, 27, 30). The inhibition of TASK channel activity by protons appears to be an effect on channel-open probability (gating) rather than blockade of the pore, and involves interaction with a histidine residue in the first pore domain, immediately following the Gly-Tyr-Gly (GYG) sequence at a site occupied by aspartate in most other K+ channels; mutation of this residue to asparagine or glutamate abolishes pH sensitivity (27, 31). Because this same histidine residue appears in both TASK channels, other determinants must account for the differential pH sensitivity of TASK-1 and TASK-3.
Pharmacological Properties of Cloned TASK Channels
At present, there are no known selective blockers of TASK channels, and identification of these channels in native contexts has relied on comparisons of properties displayed by cloned TASK channels with those of native currents. Insofar as TASK channels are unique among all cloned ion channels in generating open-rectifier K+ currents that are regulated by changes in extracellular pH, these characteristics of pH sensitivity and open rectification remain among the best diagnostic tests of TASK channel activity. Other pharmacological properties, which are not unique to TASK channels, have also been used to support evidence implicating TASK channels in native currents or to distinguish contributions from TASK-1 and TASK-3.
TASK-1 and TASK-3 generate whole-cell open-rectifier K+ currents that are very similar in terms of kinetics and voltage dependence; however, they can be distinguished from one another by the pH range over which their activity is modulated. Thus, the pK for inhibition of TASK-1 channels is ∼7.4, squarely in the physiological range, whereas the TASK-3 channel is inhibited at a more acidic pH, with a pK ∼6.7 (23–27). Simultaneous expression of both TASK-1 and TASK-3 in Xenopus oocytes leads to open-rectifier K+ currents with pharmacological properties that suggest the formation of TASK-1–TASK-3 heterodimers, with a pK intermediate between that of TASK-1 and TASK-3 (32). Although there is currently no functional evidence for native heterodimeric TASK channels, they could very well exist in a number of cell types where TASK-1 and TASK-3 are coexpressed (33, 34) ; in those cell types, a possible contribution from TASK-1–TASK-3 channels should be considered when evaluating pH sensitivity, as well as other pharmacological properties, of native TASK-like currents.
In addition to differences in pH sensitivity, TASK-1 and TASK-3 channels are also reported to be differentially sensitive to block by ruthenium red, zinc, or anandamide. Ruthenium red blocks TASK-3 at concentrations that do not inhibit TASK-1 (e.g., 5 μ M) (32, 35). The selective effects of ruthenium red on TASK-3 are attributed to a glutamate residue (Glu70 ) in the extracellular loop connecting the first membrane-spanning domain (M1) to the first pore loop (P1) of TASK-3, which is missing in TASK-1 (35). Because high-affinity blockage by ruthenium red requires that the critical glutamate residue be present in both subunits of dimeric channels (35) , TASK-1–TASK-3 heterodimeric channels have a low ruthenium red sensitivity similar to that of TASK-1 (32). Inhibition by zinc has been used to discriminate native TASK-1 from TASK-3 currents (36) , apparently based on a passing remark in one report stating that TASK-3 was unaffected by 100 μ M zinc (26) , the same concentration that was reported to inhibit TASK-1 by ∼40% in another study (25). However, in our studies of recombinant TASK channels recorded under identical conditions, we find that TASK-3 is fully inhibited by 100 μ M zinc, and moreover, concentration-response curves reveal that TASK-3 is actually more sensitive than TASK-1 to inhibition by zinc (Talley and Bayliss, unpublished observations). The endocannabinoid anandamide was also reported to be a more efficacious blocker of human TASK-1 than TASK-3 (37) , and anandamide has consequently been used to discriminate TASK-1 from TASK-3 currents in cerebellar granule neurons (38). However, this relative selectivity may be species-dependent, because rat TASK-1 and TASK-3 are approximately equally sensitive to anandamide (Talley and Bayliss, unpublished observations; Donghee Kim, personal communication), and thus, its utility for pharmacological distinction of TASK currents is questionable, at least in rat tissue. Both TASK channels are inhibited by local anesthetics, such as lidocaine and bupivacaine (25, 39). The cloned human TASK-1 channel is modestly inhibited by hypoxia (∼20% at PO2 < 40 mmHg) (40) , but to our knowledge the oxygen sensitivity of recombinant TASK-3 channels has not been described.
TASK channels are activated by clinically relevant concentrations of volatile anesthetics (39, 41, 42) , an effect which appears to be direct because TASK activation is observed in excised patches (41). Effects of anesthetics on TASK channels are sensitive to pH such that extracellular acidification prevents anesthetic-induced channel activation or inhibits TASK channels preactivated by anesthetics (42). The demonstration of a native pH- and anesthetic-sensitive K+ current with open-rectifier properties indicates the presence of functional channels in TASK-expressing neurons (42, 43). Molecular dissection of TASK channels revealed that anesthetic-dependent activation is abrogated when a six-amino-acid region (VLRFMT) at the interface of the fourth transmembrane domain and the cytoplasmic C terminus is replaced with the cognate sequence from the distantly-related TREK-1 channel (41, 44). This region is particularly interesting because molecular modeling suggests that the membrane interface is likely a key site for interactions of volatile anesthetics with membrane proteins (45) ; in addition, an analogous domain is implicated in coupling allosteric changes to gating mechanisms in other channels (46). Moreover, as described below, this same region appears also to be critical for inhibitory gating of TASK channels by receptor signaling pathways (44).
Receptor Modulation of Cloned TASK Channels
Many KCNK channels are now known to be subject to receptor-mediated modulation ( reviewed in 9–12) , and in the case of TASK channels, receptor-mediated modulation is recapitulated in heterologous expression systems through the activation of Gq/11α -coupled receptors (e.g., muscarinic M3, TRHR1 and AT1a receptors) (47–49). This class of G proteins is most commonly associated with activation of phospholipase C (PLC), and the production of two effector molecules, diacylglycerol and inositol 1,4,5-trisphosphate (IP3 ). Inhibition of TASK channels might occur through activation of phospholipase C (50 , but see 51) ; however, other commonly studied downstream signaling events do not appear to be involved in modulation of the cloned channels (11, 26, 50, 52, 53). Depletion of phosphatidylinositol bisphosphate (PIP2 ), rather than production of a specific mediator, could be responsible for TASK channel inhibition (50). This possibility has received experimental support insofar as wortmannin, which inhibits PI4-kinase activity and PIP2 regeneration, can prolong the time until recovery from receptor-mediated channel inhibition (50) ; however, confirmation of this proposed pathway would require, at least, the demonstration that TASK channels are directly modulated by PIP2.
The failure to implicate known downstream mediators in the signaling pathway between receptors and TASK channels suggests the possibility that an alternative, signaling pathway may be involved. We have found that neurotransmitter-dependent modulation of TASK1 and TASK3 requires a channel region not associated with known signaling mechanisms (44). This critical domain, located at the membrane interface, is the same region that is necessary for the activation of TASK channels by volatile anesthetics, indicating that a common molecular site is required for both increased or decreased activity of the channel (41, 44).
Surface Expression of Cloned TASK Channels
The membrane trafficking of TASK channels has been examined in heterologous expression systems (54–56). A C-terminal Ser-Ser-Val (SSV, in TASK-1) or Lys-Ser-Val (KSV, in TASK-3) motif is required for functional cell surface expression of the channels, although different proteins that bind to this domain have been identified. The p11 subunit of annexin II associates with TASK-1, but not TASK-3, in yeast two-hybrid assays (54) , and requires the SSV motif in TASK-1 for binding, as demonstrated in vitro by glutathione-S transferase (GST) pull-down assays and in vivo by coimmunoprecipitation of p11 and TASK channels expressed in COS cells. Mutations in the SSV motif of TASK-1 that disrupt p11 binding also decrease current amplitude and cell-surface expression of TASK-1. Because removal or mutation of the three basic residues (KRR) immediately adjacent to the TASK-1 C-terminal SSV motif reinstated surface expression, it was suggested that these basic residues represent the ER retention motif that is masked by p11 binding to allow transport out of the ER.
The other TASK channel binding proteins are the 14-3-3 proteins (55, 56). These phosphoserine-binding proteins, which have many putative roles in cell signaling (57) , interact with TASK channels in native and heterologous systems. The binding of 14-3-3 proteins to TASK-1 or TASK-3 depends on phosphorylation of the final C-terminal serine (i.e., only TASK-S/KSP V, but not TASK-S/KSV, can bind 14-3-3). In this case, an N-terminal dibasic ER retention motif (KR) on TASK-1 and TASK-3 binds to β -COP, a coatamer protein, in a manner that is mutually exclusive with 14-3-3 binding. Mutation of the dibasic motif promotes surface expression even in Δ SSV mutants that were incapable of binding 14-3-3 (55). These results suggest that 14-3-3 binding promotes surface expression by displacing β -COP from the N-terminal dibasic ER retention motif (55).
Despite the seemingly clear results regarding the binding of 14-3-3 or p11 to S/KSV motifs on TASK channels, two distinct ER retention sites were identified, one at the C terminus (KRR) and the other at the N terminus (KR) (54, 55). In addition, the requirement for 14-3-3 and/or p11 binding to release TASK channels from the ER does not appear absolute. For example, C-terminally truncated TASK channels that lack the p11 or 14-3-3 binding region but retain the putative N-terminal ER retention motif, can still form functional channels on the cell surface when overexpressed in a heterologous system (41, 44, 54). This suggests that the ER retention mechanism may be saturable (or leaky) or that it may involve an interaction between TASK channel N and C termini, perhaps involving the coordinated actions of different basic residue motifs identified in these two sets of studies.
Identification of Native TASK-Like Channels
Localization of TASK Channel Expression
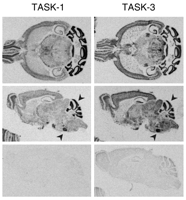
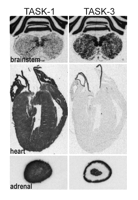
The sites of TASK channel synthesis in the CNS have been identified in various species and suggest that the expression of TASK channels is well conserved across species (12). TASK-1 is highly expressed in cerebellar granule neurons, somatic motoneurons and the locus coeruleus, whereas TASK3 is more widely distributed, with particularly high expression in somatic motoneurons, cerebellar granule neurons, the locus coeruleus and raphe nuclei, and in various nuclei of the thalamus and hypothalamus (Figure 2⇓) (33, 53, 58). Although differentially distributed, both channels are likely to be coexpressed in individual cells (e.g., cerebellar granule neurons, somatic motoneurons, locus coeruleus, thalamus), providing an anatomic basis for native TASK channel heterodimers. Outside of the CNS, TASK channel mRNA has also been identified in carotid body glomus cells (59) , a neuroepithelial body cell line (36, 60) and, as shown in Figure 3⇓, in heart (23, 24, 28, 30) and adrenal gland (34, 61).
Expression of TASK-1 and TASK-3 channels in mouse brain. Horizontal (upper) and sagittal (middle) sections of mouse brain were hybridized with [33P]-labeled cRNA antisense probes complementary to TASK-1 (left) and TASK-3 (right). As a control, sagittal sections were hybridized to the corresponding [33P]-labeled sense probes (lower). Note the differential distributions of TASK-1 and TASK-3 transcripts that also include some regions where channel expression is overlapping (arrowhead) (e.g., cerebellum and facial motoneurons).
Expression of TASK-1 and TASK-3 channels in rat brainstem, heart and adrenal gland. Sections of rat brainstem (upper), heart (middle), and adrenal gland (lower) were hybridized with [33P]-labeled cRNA antisense probes complementary to TASK-1 (left) and TASK-3 (right). High levels of TASK-1 and especially TASK-3 mRNA in hypoglossal motoneurons in rat brainstem are observed (arrows). In the rat heart, TASK-1 (but not TASK-3) is predominantly expressed. In the adrenal gland (bottom), TASK-1 transcripts are evident in all regions whereas TASK-3 transcripts are expressed at high levels in the outermost layers (zona glomerulosa and fasciculata) and in the adrenal medulla.
An understanding of the subcellular distribution of these channels would be aided by the detection of TASK channels through the use of specific antibodies. Although an antibody to TASK-1 is commercially available, it labels astrocytes strongly (47, 62) even though there is no evidence for expression of TASK-1 mRNA in those glial cells. Furthermore, this antibody gives only a faint signal in most neurons and does not label motoneurons, where TASK-1 mRNA is prominent and there is strong evidence for functional expression of TASK-1 (42, 48). Thus, the utility of that antibody in immunohistochemical studies is suspect, and reexamination with a different TASK-1 antibody, directed to a distinct epitope, may provide some resolution of the discrepant results. As yet, there has been no published description of TASK-3 channel localization in brain by immunohistochemistry.
Functional Identification of Native Neuronal TASK Channels
Consistent with expression patterns predicted from anatomical work, electrophysiological studies have identified TASK-like currents in various neuronal cell groups, including motoneurons (42, 48) , cerebellar granule cells (37, 38, 47, 63, 64) , locus coeruleus and raphe neurons (42, 43) , and magnocellular neurons of the supraoptic nucleus (65).
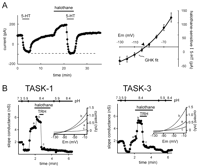
In motoneurons, we identified a pH-sensitive current with kinetic- and voltage-dependent properties virtually identical to those produced by cloned TASK channels (42, 48). These native TASK-like currents are responsible for previously described neurotransmitter- and halothane-sensitive leak K+ currents in motoneurons (7, 66) because the modulated K+ currents display open-rectifier properties and are inhibited by extracellular acidification (42, 48). In addition, neurotransmitters and anesthetics target the same pH-sensitive TASK-like channel in motoneurons, with inhibition by neurotransmitters prevailing over activation by anesthetics (67) , as observed with cloned TASK channels (Figure 4⇓). Inhibition of TASK-like channels in motoneurons involves Gq/11α -coupled receptors (48, 66) , and indeed, G proteins are implicated in transmitter-mediated leak K+ channel inhibition in motoneurons (68, 69). However, typical downstream mediators such as protein kinase C (PKC), cAMP-dependent protein kinase (PKA), IP3 , or intracellular calcium do not seem to be involved (68, 69) , and the signaling pathway leading to inhibition of the motoneuronal TASK-like current remains to be identified.
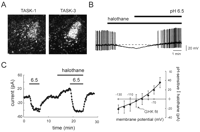
Neurotransmitters and anesthetics converge on the same TASK-like motoneuronal K+ channel and on heterologously expressed TASK-1 and TASK-3. A. (Left) In hypoglossal motoneurons, serotonin (5-HT) completely inhibits the K+ current activated by a supramaximal concentration of halothane (see dashed line). (Right) The averaged current–voltage (I-V) relationship of the joint halothane- and 5-HT-sensitive K+ current is fits well with a GHK equation, as expected for the open-rectifier TASK channels (adapted from 68). Experiments were performed with the ZD7288 (a hyperpolarization-activated cyclic-nucleotide-gated channel (HCN)-specific antagonist) in the whole cell recording pipette to block Ih, a current that is also modulated by 5-HT and halothane. B. The pH-sensitive K+ currents induced by transient transfection of TASK-1 (left) and TASK-3 (right) into TRH-R1-expressing HEK 293 cells are activated by halothane, and the halothane-activated current is completely blocked by TRH. Inset: TASK currents taken from the indicated points (a, b, c) show a weak voltage-dependence like that of the motoneuronal halothane- and 5-HT-sensitive K+ current (adapted from 44).
We detected mRNA for TASK-1 and TASK-3 and identified a K+ channel that was activated by anesthetics and inhibited by extracellular acidification in aminergic neurons of the locus coeruleus (LC) and raphe nuclei (Figure 5⇓) (42, 43). Unlike the situation in motoneurons, in which the pH-sensitive current is largely due to a TASK-like K+ conductance, it appears that multiple ionic conductances contribute to pH-sensitive currents in LC and raphe neurons. However, similar to the observations in motoneurons, halothane induces an outward current in LC and raphe neurons, and this additional halothane-sensitive current is completely suppressed in acidified bath solution. Moreover, the halothane- and pH-sensitive K+ current exhibits the voltage-dependent and kinetic properties of an open-rectifier, exactly as expected for TASK channels. In dorsal raphe neurons, the pH sensitivity of the TASK-like current is intermediate between that expected for TASK-1 and TASK-3, indicating a probable contribution from both subunits.
Locus coeruleus neurons express TASK channel transcripts and a pH- and anesthetic-sensitive open-rectifier K+ channel. A. Darkfield photomicrograph of emulsion-dipped sections hybridized with a [33P]-labeled cRNA probe specific to TASK1 (left) and TASK-3 (right) show high density of silver grains overlaying neurons in the nucleus locus coeruleus (LC) (see arrows). B. Halothane (0.3 mM) caused a membrane hyperpolarization that was completely reversed by bath acidification C. (Left) Voltage clamp recording of net membrane current in a locus coeruleus cell held at 60 mV. An acidified bath solution (pH 6.5) induced an inward shift in current and completely blocked a halothane-evoked outward current shift. (Right) Averaged data (± SEM, n=4) showing the IV relationship of the joint pH- and halothane-sensitive current in locus coeruleus neurons; this current was well fitted by using the GHK constant field equation (adapted from 44).
In cerebellar granule neurons, single channel currents exhibiting pH-sensitivity and unitary conductance similar to that of TASK-1 and TASK-3 have been described (63) and their corresponding pH-sensitive TASK-like whole cell currents recorded (37, 38, 47, 63, 64). The pH-sensitive current contributes to the previously described muscarine-sensitive standing outward-current (IK,SO ) in those neurons (47, 70). A number of additional KCNK channels are expressed in cerebellar granule neurons (e.g., TRAAK, TREK-1, and particularly, TREK-2) (33, 63) and the quantitative contribution of each subtype to the muscarine-sensitive component and/or to the overall IK,SO has not been established. As with TASK channel modulation in motoneurons and expression systems, the signaling pathway by which the activation of muscarinic receptors lead to the inhibition of IK,SO in cerebellar granule cells remains to be determined (51, 52). It is noteworthy that channel inhibition is independent of PLC activation in cerebellar neurons (52) , a situation unlike that reported for receptor-mediated inhibition of cloned TASK channels (50). Cerebellar granule neurons also possess an O2 -sensitive leak K+ current that is inhibited by extracellular acidification and anandamide, as is expected for TASK channels (38).
In addition to muscarine receptor- and O2 -mediated inhibition, another form of TASK channel modulation that occurs over a longer time scale has been observed in rat cerebellar granule cells. It appears that these neurons may have a “set point” for membrane excitability and that adjusting TASK channel expression provides a mechanism for homeostatic regulation of their intrinsic excitability. This genetic mechanism was revealed in knockout mice that do not express GABAA receptors containing α 6 and δ subunits (64). These particular receptors provide a tonic, extrasynaptic hyperpolarizing current bias in wild-type cerebellar granule neurons. The deletion of these subunits was expected to result in a relatively depolarized membrane potential in neurons from knockout mice; however, a compensatory overexpression of TASK channels provided an alternative source of tonic, hyperpolarizing current that offset the loss of the GABAA receptor current, such that neuronal excitability remained essentially unchanged. Thus, adaptive regulation of intrinsic neuronal excitability may be a feature of TASK channel expression.
Functional Identification of TASK Channels in Peripheral Tissues
Outside of the CNS, expression of functional TASK channels has been described in carotid body glomus cells (59) , lung neuroepithelial body (NEB) cells (36, 60) , cardiomyocytes (30, 71) and adrenal glomerulosa (AG) cells (34, 61).
Carotid body glomus cells and lung NEB cells are two cell types involved in oxygen chemotransduction (72) in which TASK channels are proposed to carry an O2 -sensitive K+ current (36, 60). In glomus cells, the hypoxia-inhibitable K+ current presents a number of properties of TASK channels including weak voltage-dependence, and sensitivity to pH and halothane (59). The unitary conductance of O2 -sensitive channels recorded in cell-attached patches from glomus cells is consistent with TASK-1 (14 pS) (59). In NEB-like H146 cells, hypoxia inhibits K+ currents in a pH-dependent manner, as expected for TASK currents. Although the O2 -sensitive current appears to have substantially more voltage-dependence than has been demonstrated for cloned TASK channels, antisense oligonucleotides directed against both TASK-1 and TASK-3 inhibit the O2 -sensitive K+ current in those cells (36). Hence, it seems likely that the O2 sensitivity of TASK channels is utilized in carotid body glomus and NEB cells to provide feedback to respiratory centers in the brainstem concerning the state of arterial and airway oxygenation (59, 72).
Consistent with expression of TASK-1 mRNA in the heart (Figure 3 ⇑), a 14 pS channel with kinetic properties similar to that of TASK-1 was recorded in rat atrial and ventricular myocytes (30). In addition, a weakly voltage-dependent K+ current is inhibited by platelet-activating factor (PAF) in ventricular myocytes; as expected for a current mediated by TASK channels, the ventricular PAF-sensitive current is inhibited by H+ , Zn2+ , or anandamide. Moreover, activation of an endogenous Gq/11α -coupled PAF receptor can inhibit TASK-1 channels when expressed in CHO cells (71). Unlike most other instances of receptor-mediated TASK current modulation, however, a PKC-dependent mechanism is implicated in K+ current inhibition by PAF. Regardless of the signaling pathway involved, the increased excitability in ventricular myocytes that results from PAF-mediated inhibition of the TASK-like current could contribute to the known arrythmogenic properties of PAF.
Secretion of the steroid hormone aldosterone from adrenal glomerulosa (AG) cells is provoked by elevations in serum K+ , acidosis, and by angiotensin II (AngII) (73–75). Expression of TASK-1, and particularly TASK-3, is very high in AG cells of the adrenal cortex (Figure 3) and mRNA isolated from those cells produces a weakly voltage-dependent and pH-sensitive K+ current when injected into Xenopus oocytes (34, 61). Because the majority of that current is sensitive to ruthenium red (∼75%), it seems likely that TASK-3 homodimers provide the dominant background K+ current in AG cells (34). In AG cells, membrane depolarization induced by AngII is due to AngII type 1 (AT1) receptor-mediated inhibition of a weakly-rectifying K+ current (73, 76). Correspondingly, heterologous TASK-1, TASK-3, and TASK-like currents (generated from AG cell mRNA) are all inhibited by AngII when an AT1a receptor is also coexpressed in Xenopus oocytes (34, 61). Thus, TASK channels provide a major background conductance current in AG cells, and the inhibition of these channels by H+ and AngII probably contributes to the membrane depolarization that leads to aldosterone secretion.
Physiological and Clinical Implications of TASK Channel Expression in the CNS
Role of TASK Channels in Control of Breathing and Arousal
We have established that TASK channels confer intrinsic pH sensitivity to somatic motoneurons, noradrenergic locus coeruleus neurons, and serotoninergic raphe neurons. What is the physiological consequence of this pH sensitivity? Two important neural reflexes are evoked when brain pH is decreased by hypercapnia (i.e., elevated pCO2 ): 1) enhanced respiratory output, which serves as a homeostatic mechanism to correct changes in brain pH and/or pCO2 (77) and 2) behavioral arousal, which serves a protective function when breathing is excessively depressed during sleep (78, 79).
Central respiratory chemoreceptor neurons sense changes in brain pH and CO2 and adjust the level of excitatory drive to brainstem centers that control breathing (77). Multiple brainstem sites are proposed as neural substrates for central chemoreception, and a growing literature implicates raphe serotoninergic neurons and locus coeruleus neurons in central respiratory chemosensitivity ( reviewed in 77, 80). Therefore, the pH-sensitive TASK-like currents in those cells likely contribute to central respiratory chemosensitivity (42, 43). In addition, TASK channels can mediate a direct pH-dependent increase in excitability in respiratory-related motoneurons, which ultimately convey central respiratory drive to the muscles of breathing (48). Moreover, TASK channels may contribute to enhanced peripheral feedback related to arterial pH and hypoxia that arises from carotid body and lung respiratory chemoreceptors and impinges on medullary respiratory centers (59, 72). Therefore, the pH sensitivity of TASK channels may be exploited at multiple levels to monitor changes in prevailing acid–base status for homeostatic regulation of breathing.
Behavioral arousal is also a consequence of physiological decreases in pH (78, 79). Noradrenergic LC neurons and serotoninergic raphe neurons project widely throughout the neuraxis, and their activity in vivo is tightly coupled to sleep–wake states suggesting that these cells have a key role in behavioral arousal (81). Our data suggest that modulating the activity of TASK channels will contribute to pH-dependent effects on excitability in these neurons, and thus, to modulation of arousal state. pH-sensitive TASK-dependent regulation of arousal, mediated by raphe and LC neurons, may be particularly important when ventilation becomes excessively depressed during sleep, and when the ensuing hypercapnia provokes the waking response necessary to reinstate appropriate levels of ventilation (78, 79). Activation of this arousal mechanism is responsible, at least in part, for sleep disturbances associated with obstructive apneas (82) and failure of this waking reflex in neonates might be a contributing factor to Sudden Infant Death Syndrome (SIDS) (83, 84).
Activation of TASK Channels in the Clinical Actions of Anesthetics
As mentioned above, cloned TASK channels are activated by inhalation anesthetics at concentrations that are entirely appropriate for clinically relevant anesthetic effects (39, 41) , and our results demonstrate that at these same concentrations volatile anesthetics activate a TASK-like current in somatic motoneurons, noradrenergic locus coeruleus neurons, and serotoninergic raphe neurons. At least two main effects inevitably accompany the clinical state of general anesthesia—immobilization and loss of consciousness (85) —and we have suggested that anesthetic modulation of TASK channels in these cell groups could contribute to both effects.
The immobilizing effects of volatile anesthetics are accompanied by a decrease in motoneuronal excitability, both in experimental animals and in humans (86–88). Anesthetics inhibit motoneurons, in part, by activating TASK channels in motoneurons (42) , suggesting that this mechanism can contribute directly to their immobilizing actions. In addition, because neurotransmitters elaborated from LC and raphe neurons exert facilitatory effects on motoneurons (66) , inhibition of those cell groups by anesthetic-mediated activation of TASK channels can also depress motoneuronal excitability indirectly (i.e., by disfacilitation). Thus, activation of TASK channels at a number of central sites may contribute to the immobilizing effects of anesthetics.
Raphe and locus coeruleus neurons have prominent projections to rostral brain regions and a highly state-dependent firing pattern that suggests a role in behavioral arousal (81) ; therefore, a decrease in activity of these aminergic neurons mediated by TASK channels could also contribute to the sleep-inducing effects of anesthetics (89). Consistent with this idea, LC neurons mediate hypnotic and supraspinal analgesic effects of α2 -adrenoceptor agonist anesthetic compounds such as clonidine and dexmedetomidine (90–93) , which suppress LC firing by activation of a distinct, G protein–coupled inwardly-rectifying K+ (GIRK) conductance (93). Of course, direct effects of anesthetics on other neurons expressing TASK channels (e.g., thalamic and cortical neurons) could also contribute to these and other clinically important actions of the drugs (33, 53, 58).
Conclusions
TASK channels represent a subset of the KCNK gene family that appear to be widely expressed, particularly in the CNS (33). There are no known selective blockers for TASK channels. Therefore, in TASK-expressing neurons, a contribution of these channels to native neuronal leak conductances has been determined by establishing properties that are consistent with those of their cloned counterparts (e.g., open-rectifier voltage-dependent properties and sensitivity to extracellular pH, inhalational anesthetics, and neurotransmitters). In cells where TASK-like currents are observed, it is difficult to unequivocally or quantitatively attribute native currents to TASK-1 or TASK-3, because expression patterns of TASK-1 and TASK-3 are largely overlapping, the whole cell currents produced by the individual subunits are very similar, and the subunits may heterodimerize to generate currents with intermediate pharmacological properties. It is certainly to be expected that these shortcomings may be circumvented in the future by using dominant-negative or gene knock out approaches. Nevertheless, the information on TASK channels obtained to date provides a compelling rationale for embarking on such studies, inasmuch as it suggests interesting hypotheses to test regarding physiological and/or clinical roles for these channels.
Note added in proof:
New roles for TASK channels in regulation of neuronal cell death and cell proliferation have been proposed since submission of this review. First, it was recently reported that TASK channel activity contributes to a well-known K+ -dependent apoptosis of cerebellar granule neurons (94) ; this K+ -dependent cell death was diminished by pharmacological blockers of TASK currents or by dominant negative constructs that disrupt TASK channel function. In addition, heterologous expression of TASK channels imparted K+ -dependent apoptosis onto hippocampal neurons (94). Another set of studies implicates TASK-3 as an oncogene (95, 96). The TASK-3 gene is amplified and expressed at high levels in breast cancers (95) and, accordingly, overexpression of TASK-3 in normal murine mammary gland epithelial cells (NMuMG) or C8 embryonic fibroblasts enhanced their tumorigenicity when injected into athymic nude mice (95, 96). Furthermore, TASK-3 provided anti-apoptotic effects in C8 cells cultured under serum-free or hypoxic conditions that normally induce cell death. These proliferative effects of TASK-3 expression are due to K+ channel activity since they could be blocked by a dominant negative TASK-3 channel construct (96). Thus, apparently depending on cell context, TASK channels can provide either pro- or anti-apoptotic effects, although the link between increased leak K+ channel activity and these differential effects on cell survival remain to be elucidated.
Acknowledgments
This work was supported by grants from the National Institutes of Health: NS33583 and GM66181 (D.A.B.), F32HL10271 (J.E.S.), and F31MH12091 (E.M.T.).
- © American Society for Pharmacology and Experimental Theraputics 2003
References

Douglas A. Bayliss, PhD, (left) is an Associate Professor of Pharmacology and Anesthesiology at the University of Virginia. Edmund M. Talley, PhD, (center) is an Assistant Professor of Research in the Department of Pharmacology at the University of Virginia. Jay E. Sirois, PhD, (rigth) is an Instructor in the Department of Anesthesiology at the University of Virginia.