 |
 |

A Clinical Trial of Hypertonic Saline Nasal Spray in Subjects With the Common Cold or Rhinosinusitis
Patricia Adam, MD, MSPH;
Michael Stiffman, MD, MSPH;
Robert L. Blake, Jr, MD
Arch Fam Med. 1998;7:39-43.
ABSTRACT
Objective To determine whether hypertonic saline nasal spray relieves nasal symptoms and shortens illness duration in patients with the common cold or acute rhinosinusitis.
Design Randomized trial with 2 control groups.
Setting Two family practice clinics.
Participants One hundred forty-three adult patients with a cold or sinus infection. Patients with allergic rhinitis, symptoms for more than 3 weeks, or other respiratory diagnoses were excluded, as were those who had used topical decongestants.
Intervention Hypertonic saline or normal saline spray 3 times a day or observation. Subjects completed a 7-day symptom checklist that included a well-being question ("Do you feel back to normal?").
Main Outcome Measures Nasal symptom score (sum of scores for nasal congestion, rhinorrhea, and headache) on day 3 and day of well-being (day of symptom resolution).
Results Data were collected for 119 subjects. No difference was found in either primary outcome when hypertonic saline was compared with either normal saline or observation. Mean day of well-being was 8.3 (95% confidence interval [CI], 6.9-9.7), 9.2 (95% CI, 6.9-11.43), and 8.0 (95% CI, 6.7-9.3) days in the hypertonic saline, normal saline, and observation groups, respectively. Day 3 mean nasal symptom score was 3.8 (95% CI, 3.0-4.5) for hypertonic saline, 3.7 (95% CI, 2.9-4.5) for normal saline, and 4.1 (95% CI, 3.5-4.7) for observation. Only 44% of the patients would use the hypertonic saline spray again. Thirty-two percent noted burning, compared with 13% of the normal saline group (P=.05).
Conclusion Hypertonic saline does not improve nasal symptoms or illness duration in patients with the common cold or rhinosinusitis.
INTRODUCTION
ACUTE UPPER respiratory tract infection is the fifth most common illness complex seen in the ambulatory setting, and it accounts for 2.5% of all US ambulatory visits.1 More than $2 billion is spent annually on over-the-counter medications by patients with the common cold or rhinosinusitis.2 Despite the use of decongestants, antihistamines, and occasionally antibiotics, symptoms often persist for more than 2 weeks.3
Evidence exists that some over-the-counter medications relieve nasal symptoms.4 Decongestants decrease nasal congestion.5-8 Antihistamines 9-10 and ipratropium bromide4, 11 may reduce nasal secretions, and a trend was found toward symptom relief with flunisolide corticosteroid nasal spray.12 Saline nasal spray may have decongestant properties.13-14 None of these medications, however, has been shown to alter the course of upper respiratory tract infections. The only treatment studied that probably decreases the duration of symptoms from the common cold is zinc lozenges when taken in the first 24 hours.15
There are theoretical reasons for examining whether hypertonic saline nasal spray, a 2% buffered saline solution, shortens the course of rhinosinusitis and possibly the common cold. Current theories emphasize the importance of maintaining patency of the sinus ostea and nasal ciliary function in preventing the progression from viral rhinitis to bacterial rhinosinusitis. It has been shown that, in healthy subjects, mucociliary transport improves after irrigation with hypertonic saline.16 Otolaryngologists nationwide are treating patients with chronic rhinosinusitis with hypertonic saline nasal irrigation 2 to 3 times a day to decongest the swollen membranes and to improve ciliary function.17 In the Family Practice Center at the University of Missouri-Columbia, many patients with rhinosinusitis are treated with hypertonic saline nasal spray. Its effectiveness in relieving nasal symptoms or shortening the duration of an upper respiratory tract infection, however, has never been tested. We conducted a randomized, controlled trial to study the effectiveness of hypertonic saline spray in relieving nasal symptoms and shortening their duration in patients with either the common cold or acute rhinosinusitis.
PATIENTS AND METHODS
Trained research assistants recruited subjects from 2 family practice clinics, 1 urban and 1 rural, staffed by faculty and resident physicians. The study period spanned the summer, winter, and spring of 1 year. Patients who were diagnosed by their primary care providers as having either a cold or acute rhinosinusitis were eligible for the study. We excluded patients who had duration of symptoms more than 3 weeks or a primary diagnosis of allergic rhinitis, bronchitis, or pharyngitis. We also excluded patients who had used a topical decongestant for the last 3 days or were prescribed any nasal spray medication other than topical corticosteroids. The study was approved by the institutional review board at the University of Missouri-Columbia.
Baseline data were collected by either the research assistants or the primary care provider on eligible patients who provided informed consent. A checklist of 8 symptoms (nasal congestion, rhinorrhea, postnasal drip, cough, fever, facial pressure or pain, tooth pain, and illness duration) was completed for each subject. Subjects were also examined for sinus tenderness and purulent nasal drainage. Smoking status, history of frequent rhinosinusitis, symptom relief with oral or topical decongestants, and the provider's diagnosis and treatment plan were recorded.
Subjects were randomly assigned to either the hypertonic saline nasal spray (HNS) group or 1 of 2 control groups: normal saline nasal spray (NS) or observation only. We included 2 control groups because we were concerned that normal saline might be too similar to hypertonic saline to allow us to note an effect, and comparing a nasal spray with no spray would have precluded blinding. The study pharmacist created the randomization code by means of a random number table, prepared the solutions, and packaged them in identical 2-oz spray bottles. The HNS was prepared by mixing 3 rounded teaspoons of canning/pickling salt and 1 rounded teaspoon of baking soda in 1 qt of tap water. Providers and subjects were unaware of nasal spray assignment.
Subjects in the 2 nasal spray groups were instructed to use the nasal spray 3 times a day, 2 squirts per nostril. All subjects were told to follow the provider's treatment plan (usual care) and to fill out a daily checklist for 1 week. The checklist consisted of 5 symptoms: nasal congestion, runny nose, cough, sore throat, and headache, to be rated on a 4-point severity scale (0, no symptoms; 1, mild symptoms; 2, moderate symptoms; and 3, severe symptoms). The checklist also included a question on well-being ("Are you feeling back to normal?"), on work or school absenteeism, and on the frequency of all medications taken. Subjects randomly assigned to either nasal spray group were also asked to record the frequency of nasal spray use and any side effects.
Subjects were telephoned at 3 to 4 days, 8 to 10 days, and again until symptoms resolved. The interviewer ascertained the day of well-being and inquired about compliance with nasal spray use and any nasal spray side effects. With each telephone call, subjects were reminded to return the checklist when completed.
Primary outcome measures were (1) nasal symptom score on day 3, a sum of the scores for nasal congestion, rhinorrhea, and headache for that day; and (2) day of well-being, defined as the day the subject believed that the illness had completely resolved. With day 1 being the day the subject was seen in the clinic, nasal symptom score on day 3 was chosen to evaluate early effects on nasal symptoms. Secondary outcomes studied were days of work or school missed because of illness, compliance in nasal spray use, and nasal spray side effects.
The results were analyzed according to the intention-to-treat approach, comparing the HNS group with each control group individually. Baseline characteristics, signs, symptoms, and diagnosis were compared by means of either  2 or independent t tests. For nasal symptom score on day 3, we used multivariate linear regression, adjusting for baseline symptom severity by including in the regression model the nasal symptom score on day 1 and the duration of illness before the visit. For day of well-being, we used Cox regression survival analysis and used the total symptom score on day 1 (sum of all 5 symptom scores for that day) to adjust for baseline symptom severity. Duration of illness before being seen was again controlled for. We also stratified for diagnosis (cold or rhinosinusitis) for each primary outcome measure. Potential confounders considered were antibiotic use, corticosteroid nasal spray use, over-the-counter medication use, smoking, history of frequent rhinosinusitis, and duration of illness before being seen in the clinic. Side effects and medication use were compared with 2 or independent t tests. For nasal symptom score on day 3, we used multivariate linear regression, adjusting for baseline symptom severity by including in the regression model the nasal symptom score on day 1 and the duration of illness before the visit. For day of well-being, we used Cox regression survival analysis and used the total symptom score on day 1 (sum of all 5 symptom scores for that day) to adjust for baseline symptom severity. Duration of illness before being seen was again controlled for. We also stratified for diagnosis (cold or rhinosinusitis) for each primary outcome measure. Potential confounders considered were antibiotic use, corticosteroid nasal spray use, over-the-counter medication use, smoking, history of frequent rhinosinusitis, and duration of illness before being seen in the clinic. Side effects and medication use were compared with  2. We defined P<.05 as statistically significant. Both primary outcome measures were also compared nonparametrically by means of the Wilcoxon rank-sum test. 2. We defined P<.05 as statistically significant. Both primary outcome measures were also compared nonparametrically by means of the Wilcoxon rank-sum test.
RESULTS
We approached 324 patients; 49 patients chose not to participate. Forty-six were excluded because of symptoms present for more than 3 weeks; 31 for symptoms caused by allergies; 17 for topical decongestant use; 13 for diagnosis other than cold or rhinosinusitis; and 19 for nasal spray prescription, chronic sinus disease, or other. Of the 149 subjects enrolled, 6 subjects (3 in the HNS group and 3 in the NS group) were excluded after randomization because of respiratory illnesses other than cold or rhinosinusitis, or missed exclusion criteria. Thus, 143 patients (48 in the HNS group, 46 in the NS group, and 49 in the observation group) were studied. Of these subjects, 72% were referred by faculty and third-year residents; no 1 provider was primarily responsible for recruitment.
We collected outcome data on 119 subjects, of whom 84 returned a completed symptom checklist and 113 reported a day of well-being. Despite numerous attempts at contacting them, 24 subjects were completely unavailable for follow-up, and another 25 checklists were lost or not returned. The dropout rate was similar in all 3 groups, as were the baseline characteristics when those unavailable for follow-up and excluded after randomization were compared with those who completed the study.
Subjects were predominately female (69%) and nonsmokers (77%), with a mean age of 37 years. Two thirds were diagnosed as having rhinosinusitis by their providers. Review of all the clinic visits during the study periods showed that we were twice as successful at recruiting subjects with rhinosinusitis. Randomization resulted in similar baseline characteristics, baseline signs and symptoms, and diagnosis in all 3 groups (Table 1) except for the presence of sinus tenderness, which was more common in the HNS group than in the observation group. Mean total symptom score on day 1, a marker of symptom severity at baseline, was similar in all the groups: 7.8 (95% confidence interval [CI], 6.7-8.9) in the HNS group, 6.6 (95% CI, 5.7-7.5) in the NS group, and 8.4 (95% CI, 7.3-9.5) in the observation group. Primary care providers were much more likely to diagnose rhinosinusitis if any of the following were present: facial pressure or pain, history of purulent drainage, purulence visualized, sinus tenderness to percussion (all P<.001), or maxillary tooth pain (P<.01).
|
|

|
|
Table 1. Baseline Characteristics*
|
|
|
Since diagnosis of rhinosinusitis was evenly distributed among the 3 groups, there was little variation in antibiotic use. Ninety-eight percent of subjects diagnosed as having rhinosinusitis were treated with an antibiotic, compared with 18% of subjects diagnosed as having a cold. Sixteen percent of the subjects in the observation group were prescribed corticosteroid nasal sprays, compared with 7% in the HNS group (P=.2) and 9% in the NS group. Use of any over-the-counter medication (pain relievers, decongestants, antihistamines, or combination formulations) during the first week was 77% in the HNS group compared with 86% (P=.38) and 83% (P=.56) in the NS and observation groups, respectively. No significant differences were found with respect to the primary outcome measures when HNS was compared with either control group. Mean day of well-being was 8.3 (95% CI, 6.9-9.7), 9.2 (95% CI, 6.9-11.43), and 8.0 (95% CI, 6.7-9.3) in the HNS, NS, and observation groups, respectively. One subject in the NS group had a much longer duration of illness (35 days). With this outlier excluded, the mean day of well-being for the NS group was 8.3 (95% CI, 6.8-9.8). Including or excluding this subject, survival analysis with Cox regression to control for total symptoms on day 1 and duration of illness before initial examination showed no difference between HNS and observation (P=.24) and HNS and NS (P=.93) (Figure 1). Mean nasal symptom score on day 3 was 3.8 (95% CI, 3.0-4.5), 3.7 (95% CI, 2.9-4.5), and 4.1 (95% CI, 3.5-4.7) in the HNS, NS, and observation groups, respectively. Mean nasal symptom scores for all days of follow-up were similar in the 3 groups (Figure 2). These results were unchanged after multivariate analysis and after nonparametric analyses with the Wilcoxon rank-sum test. There were no differences in work days missed because of illness among the groups.
|
|

|
|
Figure 1. Recovery curves showing the day subjects felt "back to normal." One subject in the normal saline group with a value of 35 days was excluded.
|
|
|
|
|

|
|
Figure 2. Comparison of nasal symptom scores.
|
|
|
Stratifying by diagnosis of rhinosinusitis or cold did not affect the results for either primary outcome variable, although definitive assessment of the effect on subjects with colds was limited by small numbers. Figure 3 depicts the effect of stratification on the day of well-being.
|
|

|
|
Figure 3. Recovery curves showing the day subjects felt "back to normal" by diagnosis. One subject in the normal saline group with a value of 35 days was excluded. HNS indicates hypertonic saline; NS, normal saline.
|
|
|
Assessment for a possible type II error by means of post hoc power calculations demonstrated a power of 98% to detect a 50% reduction in mean nasal symptom score on day 3, and a power of 37% to detect a 25% reduction in mean score. Our study had a power of 69% to detect a 25% decrease in length of illness (difference of 2 days) and a power of 28% to detect a 12.5% decrease (difference of 1 day). Fewer than half the subjects would use the nasal spray again (Table 2). In the HNS group, those with colds were more likely to use the nasal spray again (P=.007). Thirty-two percent of those assigned to HNS noted nasal irritation, compared with 13% of those using NS (P=.05). Also, those using HNS were somewhat more likely to stop using the spray, with a quit rate of 17% on day 4 compared with 6% for NS (P=.14). Nine individuals in the HNS group, however, volunteered that they noticed a positive effect (usually improved nasal drainage), compared with 5 NS users, while 11 HNS users noted no effect or only a fleeting effect, compared with 14 in the NS group. Uncommon side effects volunteered by those assigned to nasal sprays included local burning and nausea caused by drainage (Table 2).
|
|

|
|
Table 2. Nasal Saline Spray Side Effects
|
|
|
These results provide information about the natural course of upper respiratory tract infections in adults who seek care from primary care providers. Considering the total length of illness, both before and after the clinic visit, half of the subjects felt back to normal by 15.5 days. Those diagnosed as having rhinosinusitis were sick longer, with median illness duration of 18.5 days compared with 13 days for those diagnosed as having colds. This 5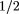 -day difference is partially explained by the fact that, at the time of diagnosis, patients with rhinosinusitis reported being ill 2 days longer than patients with colds. From the time patients came for care, half of all subjects were well in 7.9 days: 8.3 days for those with rhinosinusitis and 7.6 days for those with colds. -day difference is partially explained by the fact that, at the time of diagnosis, patients with rhinosinusitis reported being ill 2 days longer than patients with colds. From the time patients came for care, half of all subjects were well in 7.9 days: 8.3 days for those with rhinosinusitis and 7.6 days for those with colds.
COMMENT
This trial found that hypertonic nasal saline spray has no measurable effect on duration or severity of nasal symptoms in patients with the common cold or rhinosinusitis. The mean time until resolution of symptoms was approximately 8 days, regardless of treatment, with two thirds of subjects requiring other medication for symptom relief. Less than half of the subjects would use the nasal spray again, with a third of all subjects noting nasal irritation and several perceiving a lack of effect. A few subjects, however, reported improved drainage and were very satisfied with the HNS.
Other studies of upper respiratory tract illnesses have found a similar duration of symptoms. The study by Holleman et al3 of subjects with sinus complaints whose sinus x-rays were negative for rhinosinusitis noted a median time to "clinical success" of 14 days. The control group in the study by Mossad et al15 of zinc lozenges had a median time until symptom resolution of 7.6 days. We were somewhat surprised to discover that the patients with rhinosinusitis were sick longer than those with colds. One study of x-ray–positive subjects with rhinosinusitis found the median time to clinical success to be approximately 4 days.18 Our providers may have diagnosed rhinosinusitis more frequently in patients who were more ill, although baseline total symptom scores in those diagnosed as having rhinosinusitis were no different from scores in those diagnosed as having colds.
A strength of our study is its applicability. We deliberately designed our trial to resemble current practice. Most primary care providers distinguish between acute rhinosinusitis and the common cold on the basis of the history and physical findings and do not use x-ray or laboratory evaluation. Physicians' clinical impressions compare favorably with x-ray and culture data.19-20 In our study, diagnosis of rhinosinusitis correlated strongly with signs and symptoms previously found to be specific for rhinosinusitis. This was true despite the many providers who referred subjects into the study. Also, our goal was to study the effect of HNS on nasal symptoms in those with upper respiratory tract infections, whether bacterial or viral. By focusing on symptoms rather than pathophysiologic characteristics, we were able to show that HNS is probably not beneficial in patients with acute nasal congestion and rhinorrhea from infectious causes.
Limitations of our study include the possibility of a type II error. The power to detect a clinically significant difference in the day of well-being is not particularly high. It is possible that a study with more subjects might detect a clinically significant decrease in duration of illness, although our finding of a slight prolongation of illness in the HNS group compared with the observation group makes a beneficial effect less likely. Also, our study did find a clinically and statistically significant increase in side effects from the use of HNS and that most patients would probably not use the spray again.
Our study is also limited by incomplete recruitment and follow-up. It is possible that those who refused to participate and those who did not return the checklists or were unavailable for follow-up were more likely to respond favorably to the HNS treatment. There is no reason, however, to believe that this is the case; baseline characteristics were similar for subjects who completed the study and those who did not. This diminishes the likelihood of a response bias.
Despite the limitations, we believe these results are of value to physicians who provide care to adults with upper respiratory tract symptoms. This trial found no evidence that use of HNS has a beneficial effect on symptoms of the common cold or acute rhinosinusitis. The majority of subjects were reluctant to use the nasal spray again, primarily because of perceived lack of effect.
AUTHOR INFORMATION
Accepted for publication December 16, 1996.
Corresponding author: Patricia Adam, MD, MPH, Riverside University Family Practice Clinic, University of Minnesota Department of Family Practice and Community Health, 2615 E Franklin Ave, Minneapolis, MN 55406 (e-mail: padam{at}famprac.umn.edu).
From the Riverside University Family Practice Clinic (Dr Adam) and St Paul Family Medicine Residency Program/Healthpartners (Dr Stiffman), University of Minnesota Department of Family Practice and Community Health, Minneapolis, and Department of Family and Community Medicine, University of Missouri-Columbia (Dr Blake).
REFERENCES
1. Principal diagnosis and ICD-9-CM Code. Vital Health Stat. 1993-1994;series 13:114-116.
2. National Disease and Therapeutic Index. Plymouth Meeting, Pa: IMS Inc; 1989:487.
3. Holleman DR, Williams JW, Simel DL. Usual care and outcomes in patients with sinus complaints and normal results of sinus roentgenography. Arch Fam Med. 1995;4:246-251.
FREE FULL TEXT
4. Smith MBH, Feldman W. Over-the-counter cold medications. JAMA. 1995;269:2258-2263.
5. Sperber SJ, Sorrentino JV, Riker DK, Hayden FG. Evaluation of an alpha agonist alone and in combination with a nonsteroidal antiinflammatory agent in the treatment of experimental rhinovirus colds. Bull N Y Acad Med. 1989;65:145-160.
WEB OF SCIENCE
| PUBMED
6. Darmansjah I, Akib T, Setiawati A, Muchtar A, Rifki N. A dose-ranging study of phenylpropanolamine on nasal airflow. Int J Clin Pharmacol Ther Toxicol. 1990;28:282-285.
PUBMED
7. Akerlund A, Klint T, Olen L, Rundcrantz H. Nasal decongestant effect of oxymetazoline in the common cold: an objective dose-response study in 106 patients. J Laryngol Otol. 1989;103:743-746.
WEB OF SCIENCE
| PUBMED
8. Riker DK, Hendley JO, Doyle WJ, et al. Therapeutic effects of an anticholinergic-sympathomimetic combination in induced rhinovirus colds. Ann Otol Rhinol Laryngol. 1993;102:521-527.
WEB OF SCIENCE
| PUBMED
9. Doyle JF, McBride TP, Skoner MP, Maddern BR, Swaltney JM, Uhrin M. A double-blind, placebo-controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear and eustachian tube to provocative rhinovirus challenge. Pediatr Infect Dis J. 1988;7:229-238.
WEB OF SCIENCE
| PUBMED
10. Howard JC, Kantner TR, Litienfield LS, et al. Effectiveness of antihistamines in the symptomatic management of the common cold. JAMA. 1979;242:2414-2417.
FREE FULL TEXT
11. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds. Ann Intern Med. 1996;125:89-97.
FREE FULL TEXT
12. Meltzer EO, Orgel HA, Backhaus JW, et al. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. J Allergy Clin Immunol. 1993;92:812-823.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
13. Druce HM. Adjuncts to medical management of sinusitis. 1990;103:880-883.
14. Slavin RG. Management of sinusitis. J Am Geriatr Soc. 1991;39:212-217.
WEB OF SCIENCE
| PUBMED
15. Mossad SB, Macknin ML, Medendorp SV, Mason P. Zinc gluconate lozenges for treating the common cold. Ann Intern Med. 1996;125:81-88.
FREE FULL TEXT
16. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope. 1997;107:500-503.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
17. Parsons DS. Chronic sinusitis: a medical or surgical disease? Otolaryngol Clin North Am. 1996;29:1-9.
WEB OF SCIENCE
| PUBMED
18. Williams JW, Holleman DR, Samsa GP, Simel DL. Randomized controlled trial of 3 vs 10 days of trimethoprim-sulfamethoxazole for acute maxillary sinusitis. JAMA. 1995;273:1015-1021.
FREE FULL TEXT
19. Williams JW, Simel DL, Roberts L, Samsa G. Clinical evaluation for sinusitis. Ann Intern Med. 1992;117:705-710.
20. Berg O, Bergstedt H, Lind MG, Carenfelt C, Perols O. Discrimination of purulent from nonpurulent maxillary sinusitis. Ann Otol. 1981;90:272-275.
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
Rhinosinusitis Diagnosis and Management for the Clinician: A Synopsis of Recent Consensus Guidelines
Meltzer and Hamilos
Mayo Clin Proc. 2011;86:427-443.
ABSTRACT
| FULL TEXT
Efficacy of Isotonic Nasal Wash (Seawater) in the Treatment and Prevention of Rhinitis in Children
Slapak et al.
Arch Otolaryngol Head Neck Surg 2008;134:67-74.
ABSTRACT
| FULL TEXT
The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients
Hauptman and Ryan
Otolaryngol Head Neck Surg 2007;137:815-821.
ABSTRACT
| FULL TEXT
Nasal Saline for Chronic Sinonasal Symptoms: A Randomized Controlled Trial
Pynnonen et al.
Arch Otolaryngol Head Neck Surg 2007;133:1115-1120.
ABSTRACT
| FULL TEXT
Clinical practice guideline: Adult sinusitis
Rosenfeld et al.
Otolaryngol Head Neck Surg 2007;137:S1-S31.
ABSTRACT
| FULL TEXT
Nasal Irrigation for the Alleviation of Sinonasal Symptoms
Heatley et al.
Otolaryngol Head Neck Surg 2001;125:44-48.
ABSTRACT
| FULL TEXT
A Clinical Trial of Hypertonic Saline Nasal Spray
Carlston et al.
Arch Fam Med 1999;8:100-100.
FULL TEXT
DOES HYPERTONIC SALINE NASAL SPRAY RELIEVE COLD SYMPTOMS?
JWatch General 1998;1998:6-6.
FULL TEXT
|